Despite their danger, chlorates are still produced on an industrial scale and play an important, mostly unnoticed role in our day-to-day life. This merits a closer look. Regardless, it remains true that there were many chlorate explosions along the way, as Claude Louis Berthollet discovered at the end of the 18th century.
2 Chlorate Explosions Pave the Way
2.1 Berthollet’s Synthesis of Potassium Chlorate
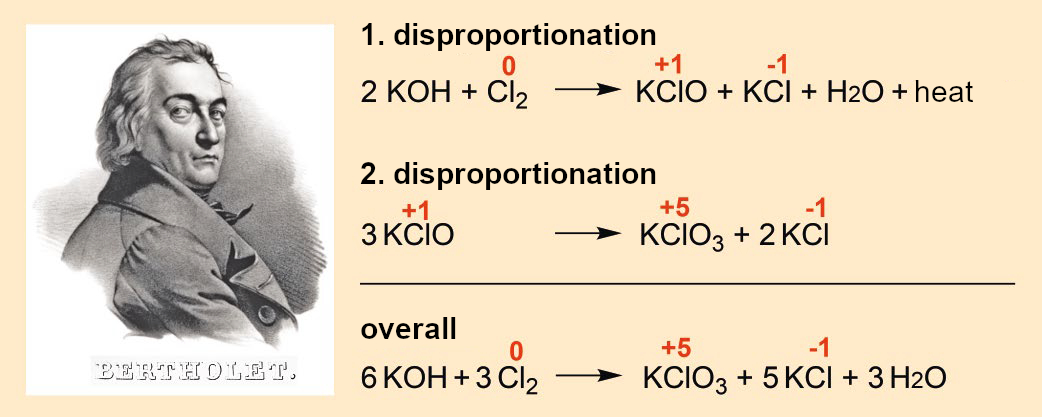
The first chlorate, potassium chlorate, was discovered by Claude Louis Berthollet (1748–1822) in 1788 [15,16]. By introducing elemental chlorine to an aqueous potassium hydroxide (KOH) solution and then evaporating it, he obtained potassium chlorate (KClO3) for the first time (see Fig. 3):
“I poured a concentrated solution of potash into a bottle and introduced a lot of chlorine. Considerable amounts of the gas were absorbed. The solution became cloudy and large amounts of a solid precipitated. […] Several crystals of a new form were also formed. I evaporated the liquid. I obtained a large amount of potassium chloride and a small amount of a new salt.”
Initially, the elemental chlorine disproportionates in Reaction 1 (oxidation numbers in red) to form chloride and hypochlorite (KClO). In concentrated solutions, this exothermic reaction leads to such a great increase in temperature that a second disproportionation reaction (Reaction 2) occurs, forming the chlorate.
To make it easier to understand, Berthollet’s experiment is described here with modern nomenclature. At the end of the 18th century, it was not yet known that chlorine and potassium were elements [17].
 |
|
Figure 3. Berthollet‘s synthesis of potassium chlorate (1788); image source: Wellcome Images, wikimedia commons, CC BY 4.0. |
Berthollet discovered that potassium chlorate gave off more oxygen than potassium nitrate upon heating, and that the new salt exploded very easily in mixtures with other substances:
“It detonates with carbon. – After the explosion, all that remains is normal potassium chloride. This experiment proves that it consists of chlorine, oxygen, and potassium.”
2.2 Potassium Chlorate and Gunpowder
The extremely high explosive power of the potassium chlorate/carbon mixture impressed Berthollet. He saw an opportunity to improve on traditional gunpowder [18,19] by replacing the potassium nitrate with potassium chlorate.
Berthollet proposed his idea to Antoine Laurent de Lavoisier (1743–1794)—one of the Directors (Régisseurs) of the French Gunpowder Commission (Régie de Poudre). In his position of leadership, Lavoisier had improved the quality of gunpowder to such an extent that French gunpowder was the best in the world. It was no wonder that Lavoisier was excited about Berthollet’s idea and immediately ordered the production of a large amount of potassium chlorate in the Paris arsenal. By trial and error with small samples, they determined that the most explosive mixture consisted of 75 % potassium chlorate, 10 % sulfur, and 5 % charcoal [20].
They planned their first major test for Sunday, October 27, 1788, at six in the morning at the state gunpowder factory in Essonne. On the day before the test, a coach brought Lavoisier, Lavoisier’s wife, Berthollet, and the Director of the gunpowder factory, M. Le Tort, from Paris to Essonne [21]. For this first experiment, the Director had a special powder mill built outdoors. It consisted of a large mortar and a pestle that was operated by a crank handle with a shaft that passed through a protective wall made of thick wooden planks. This allowed the pestle to be handled safely.
Eight people were present: In addition to the Lavoisiers, Berthollet, and Director Le Tort, the group included a member of the Gunpowder Commission, M. Chevraud, his young sister Miss Chevraud, and two employees of the gunpowder factory.
First, sixteen pounds of the three ingredients (charcoal, sulfur, and potassium chlorate) were placed in the mortar. For safety, the mixture was moistened with water. Once the grinding began, difficulties arose because the wet mass stuck to the pestle. Le Tort became impatient. He added another four pounds of the dry mixture and kept scraping the powder mass off the pestle—when warned, he argued that there was no danger yet because the powder was not fully mixed.
Lavoisier was angry at the Director’s recklessness and insisted that everyone stay behind the wooden safety wall while the mill was in operation. Once the mixture appeared to be homogenous, they paused to enjoy breakfast in the nearby factory building. In the meantime, the mortar was guarded by the two factory workers, and Lavoisier directed them to remain behind the wooden safety barrier.
After breakfast, the group strolled back with Le Tort and Miss Chevraud in front and the rest following at some distance. As the first two reached the mortar, there was a powerful explosion. The Lavoisiers, Berthollet, and M. Chevraud rushed in to help and saw the shattered mortar. The pestle had flown a great distance, and Le Tort and Miss Chevraud had been thrown about nine meters through the air against a wall made of millstones. The young woman died immediately, and Director Le Tort died a half hour later. The two factory workers had followed Lavoisier’s orders and remained behind the wooden safety wall. The workers, the Lavoisiers, Berthollet, and M. Chevraud escaped with only a scare.
This tragic accident ended French attempts to improve their gunpowder with potassium chlorate. However, it was not the end for chlorates: Over the next two centuries, they were used as powerful oxidizing agents in a variety of applications, such as in the heads of safety matches, as the basis of many fireworks [22,23], as versatile oxidizing agents in the pigment industry, as a disinfectant in medical settings, and as a broad-spectrum herbicide in agriculture.
Potassium and sodium chlorate are chemically very similar. Both salts are referred to by the term “chlorates”. One difference between them is their water solubility at 20 °C (NaClO3: 916 g/L; KClO3: 73 g/L). Unlike potassium chlorate, sodium chlorate is hygroscopic, making it unsuitable for use in matches and fireworks.
3 Chlorate Accidents
Today, 4.5 million tons of sodium chlorate are produced electrolytically every year around the world [24]. The areas of application have shifted significantly in the last 200 years. Most of it is used to produce chlorine dioxide, which is employed as a bleach in the paper industry and in the treatment of drinking water [25]. The use of chlorates in fireworks is declining because potassium chlorate has largely been replaced by perchlorates, which are easier to handle. The importance of chlorates as herbicides has decreased greatly and their use for this purpose has not been allowed in the EU since 2008 [26].
Though data is sparse, it can be assumed that more accidents like the tragic 1788 explosion in Essonne have occurred over the last 200 years [27–30]. Searching for the term “potassium chlorate” on YouTube raises the fear that some content will cause kids, with their curiosity and excitement about explosions, to (inappropriately!) imitate the videos.
In a 1956 U.S. analysis of chemistry accidents involving youth between 13 and 17 years of age, 92 % of the accidents were explosions, and 36 % involved potassium chlorate. At the time, potassium chlorate was the most dangerous chemical in the hands of children, and the results of the accidents were devastating [31]: 26 % fatalities, 23 % severe injuries, 16 % slight injuries, 12 % loss of one or both hands, 12 % loss of one or more fingers, and 10 % severe burns.
We do have to account for the fact that in the USA and Europe in the 1950s, many chemicals were more or less freely available. In Germany, for example, it was well known that the weedkillers popular at the time, UnkrautEx (in the Federal Republic of Germany) and Wegerein K (in the German Democratic Republic) were pure chlorates. Fortunately, it has been illegal to provide potassium chlorates to private individuals in the EU since February 1, 2021 [32]. Hopefully, this will prevent the inappropriate use of potassium and sodium chlorate by youth in the future, especially on New Year’s Eve.
References
[15] M. Berthollet, Observations sur la physique de l’abbé Rozier 1788, 33, 217.
[16] P. Lemay, Berthollet invente des explosifs, Rev. Hist. Pharm. 1961, 169, 53–57.
[17] P. Lemay, R. E. Oesper, Claude Louis Berthollet (1748–1822), J. Chem. Educ. 1946, 23, 158. https://doi.org/10.1021/ed023p158
[18] F. Seel, Geschichte und Chemie des Schwarzpulvers. Le charbon fait la poudre, Chem. Unserer Zeit 1988, 22, 9–16. https://doi.org/10.1002/ciuz.19880220103
[19] K. W. Böddeker, Zur Kulturgeschichte der Explosivstoffe: Konstruktiv, destruktiv, Chem. Unserer Zeit 2001, 35, 382–388. https://doi.org/10.1002/1521-3781(200112)35:6<382::AID-CIUZ382>3.0.CO;2-N
[20] M. Antelman, Lecture demonstrations of incendiaries. II, J. Chem. Educ. 1955, 32, 273. https://doi.org/10.1021/ed032p273
[21] C. C. Gillispie, Science and Polity in France: The End of the Old Regime, Princeton University Press, Princeton, USA, 1980. ISBN: 9780691118499
[22] K. Menke, Die Chemie der Feuerwerkskörper, Chem. Unserer Zeit 1978, 12, 12–22. https://doi.org/10.1002/ciuz.19780120103
[23] F. Keller, Feuerwerk: Von der Chemie zur Show, Chem. Unserer Zeit 2012, 46, 248–265. https://doi.org/10.1002/ciuz.201200596
[24] Global Sodium Chlorate Market Outlook, www.expertmarketresearch.com (accessed August 24, 2022)
[25] H. Vogt et al., Chlorine Oxides and Chlorine Oxygen Acids, Ullman’s Encyclopedia of Industrial Chemistry 2010. https://doi.org/10.1002/14356007.a06_483.pub2
[26] EU Amtsblatt L307/7/2008/865/EG, Entscheidung der Kommission vom 10. November 2008 über die Nichtaufnahme von Chlorat in Anhang I der Richtlinie 91/414/EWG des Rates und die Aufhebung der Zulassungen für Pflanzenschutzmittel mit diesem Stoff, November 18, 2008.
[27] F. E. Rowland, Laboratory and Plant: An Unusual Explosion in Connection With Potassium Chlorate, J. Ind. Eng. Chem. 1916, 6, 517–518. https://doi.org/10.1021/i500006a015
[28] F. E. Brown et al., The Decomposition of Potassium Chlorate. I. Spontaneous Decomposition Temperatures of Mixtures of Potassium Chlorate and Manganese Dioxide, J. Am. Chem. Soc. 1923, 45, 1343–1348. https://doi.org/10.1021/ja01659a001
[29] Failure and Accidents Technical information System (FACTS), Chemical Accidents with Potassium Chlorate, www.factsonline.nl (accessed August 24, 2022)
[30] vanceb1, Sodium Chlorate Explosion, youtube.com, 2012. (accessed August 24, 2022)
[31] C. Burns, Chemical accidents involving minors, J. Chem. Educ. 1956, 33, 508. https://doi.org/10.1021/ed033p508
[32] Official Journal of the European Union, Regulation (EU) 2019/1148 of the European Parliament and of the Council of 20 June 2019 on the marketing and use of explosives precursors, amending Regulation (EC) No 1907/2006 and repealing Regulation (EU) No 98/2013, July 11, 2019.
The article has been published in German as:
- Als Richard Buckleys Hose explodierte,
Klaus Roth,
Chem. unserer Zeit 2022, 56, 102–109.
https://doi.org/10.1002/ciuz.202100062
and was translated by Caroll Pohl-Ferry.
Chlorates: Tragic Incidents and Life-Saving Applications – Part 1
A tale of exploding trousers, herbicides, fireworks, and airplane oxygen masks
Chlorates: Tragic Incidents and Life-Saving Applications – Part 3
A vital use of chlorates as a source of oxygen—for example, in airplanes
See similar articles by Klaus Roth published on ChemistryViews.org