The conversion of alkanes to alkenes is an important type of transformation, both in organic synthesis in the lab and in the industry. For example, transition-metal catalysis can be used to dehydrogenate carbonyl compounds and obtain conjugated products. The formation of E-alkenes is usually thermodynamically preferred. Z-alkenes can be challenging to access, and dehydrogenation methodologies to selectively form Z-alkenes had not been reported so far.
Timothy R. Newhouse, Yale University, New Haven, CT, USA, and colleagues have developed a Z-selective, cobalt-catalyzed propargylic dehydrogenation that gives 1,3-enynes (pictured). The team used CoCl2 as a catalyst, PMe3 as a ligand, pyridine as an additive, Zn(tmp)2 (tmp = 2,2,6,6-tetramethylpiperidinyl) as a base, 2-bromo-3-methylthiophene as an oxidant, as 2-MeTHF as the solvent. The reactions were performed at 75 °C.
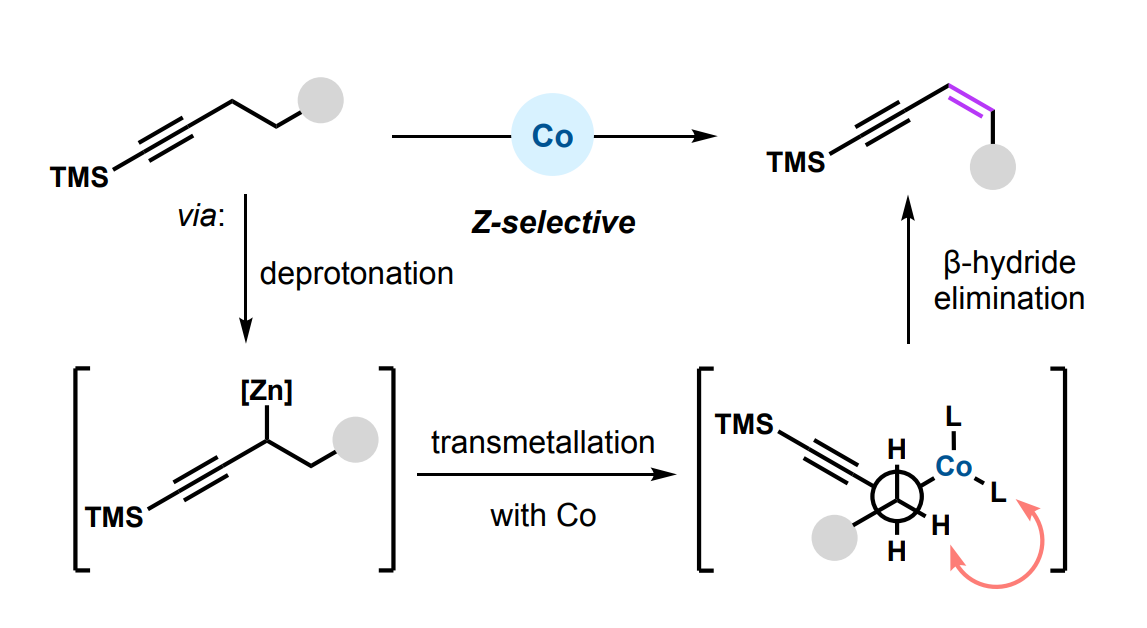
Under these conditions, a variety of 1,3-enynes was obtained in moderate to high yields and with a preference for the Z-isomers. The team proposes that the Z-selectivity could stem from the minimization of steric hindrance between the ligand sphere at the cobalt center and the substrate during the selectivity-determining step, a β-hydride elimination (pictured above). According to the researchers, the work represents the first report of a transition-metal mediated dehydrogenation at a propargylic position and the first report of a transition-metal dehydrogenation to favor the Z-isomer of the alkene.
- Accessing Z‐Enynes via Cobalt‐Catalyzed Propargylic Dehydrogenation,
Alexandra K. Bodnar, Timothy R. Newhouse,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202402638