Wacker-type reactions can be used to oxidize olefins to ketones, and they are usually catalyzed by palladium complexes. For example, in industry, acetaldehyde is most often synthesized from ethene using a Wacker process. To replace expensive palladium, iron catalysts such as iron porphyrinoids have been developed. When producing ketones, however, µ-oxo-bridged diiron species may limit productivity, and the reactions require high catalyst loadings and long reaction times.
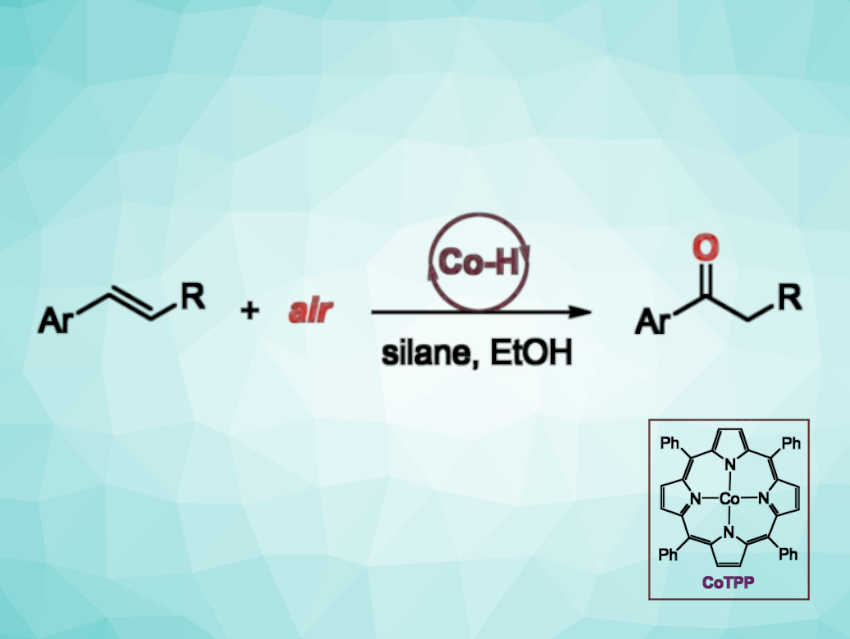
Rafael Gramage-Doria, Rennes University, France, Andreas W. Ehlers, Bas de Bruin, University of Amsterdam, The Netherlands, and colleagues have found that a cobalt-tetraphenylporphyrin (CoTPP) complex can catalyze the oxidation of styrenes to ketones in air (pictured), with silanes acting as a hydrogen source. The team transformed a variety of styrene derivatives in the presence of CoTPP and Et3SiH, using ethanol as the solvent. The reactions were performed at room temperature within 10 min.
The desired ketone products were obtained in high to excellent yields. The ketones are formed selectively over alcohols and aldehydes. The reaction tolerates fluoro, chloro, bromo, methoxy, and ester-substituted aromatic olefins.
Based on density functional theory (DFT) calculations, the researchers propose a reaction mechanism that involves the formation of a cobalt-hydride species, followed by a transfer of the cobalt-coordinated hydrogen atom to the terminal carbon atom of the olefin. The resulting close-contact radical reacts with molecular oxygen, leading to a peroxo Co(III) complex. One of the oxygen atoms becomes part of the ketone product, the other remains part of a Co–OH group. Et3SiH then regenerates the CoTPP hydride.
- Markovnikov‐Selective Cobalt‐Catalyzed Wacker‐Type Oxidation of Styrenes into Ketones under Ambient Conditions Enabled by Hydrogen Bonding,
Naba Abuhafez, Andreas W. Ehlers, Bas de Bruin, Rafael Gramage‐Doria,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202316825