Cobaltocenium salts are sandwich complexes of cobalt(III) and two cyclopentadienide anions, similar to the well-known iron-containing ferrocene derivatives. Ferrocene can be functionalized in various ways and its derivatives are useful for many applications, for example, as parts of ligand structures which can coordinate other metals and facilitate catalytic reactions. Cobaltocenium derivatives, whose electronic properties differ from those of ferrocene due to their cationic character, are also promising materials for such applications but this requires efficient functionalization pathways.
Maren Podewitz, TU Wien, Vienna, Austria, Benno Bildstein, Universität Innsbruck, Austria, and colleagues have developed a synthesis route for cobaltocenium carbaldehyde (formylcobaltocenium) and tested its reactivity in carbonyl-specific reactions. Their synthesis started with cobaltocenium carboxylic acid hexafluorophosphate, which was transformed to the acid chloride using thionyl chloride. The chloride was then reduced to the corresponding alcohol by bis-(triphenylphosphine)copper(I) tetrahydridroborate. Finally, the alcohol was oxidized to the aldehyde using Dess-Martin periodinane (pictured below).
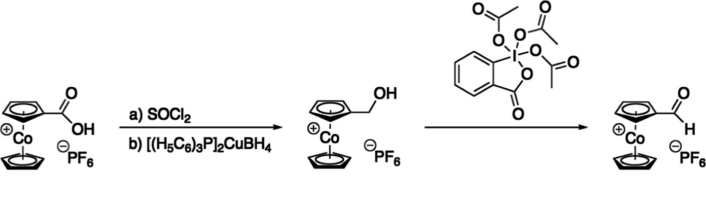
The researchers were able to produce cobaltocenium carbaldehyde hexafluorophosphate with an overall yield of 38 %. They characterized the aldehyde using several methods, including 1H and 13C-NMR spectroscopy, infrared spectroscopy, and singly crystal X-ray diffraction.
The team also tested the reactivity of the aldehyde in typical aldehyde condensation reactions. They found that the complex does not react with nucleophiles to form, for example, Schiff bases, as organic aldehydes do. Instead, they observed cleavage of the formyl group, which they attributed to the strong electron-withdrawing effect of the cobaltocenium substituent with its cationic metal center. Furthermore, during cyclovoltammetry, the aldehyde underwent reversible reduction to the neutral Co(II) complex. This reduction was chemically achieved by KC8-reduction under strictly inert conditions. The additional electron is located at the cobalt center, providing different reactivity than typical formyl-radicals.
In summary, cobaltocenium carbaldehyde hexafluorophosphate is easily accessible but its reactivity strongly differs from that of typcial aldehydes.
- Curious Case of Cobaltocenium Carbaldehyde,
Daniel Menia, Michael Pittracher, Holger Kopacka, Klaus Wurst, Florian R. Neururer, Daniel Leitner, Stephan Hohloch, Maren Podewitz, Benno Bildstein,
Organometallics 2023.
https://doi.org/10.1021/acs.organomet.2c00613