Helicenes are fully conjugated molecules with helical structures that can show intense responses in chiroptical spectroscopy, such as in circular dichroism (CD) and circular polarized luminescence (CPL) spectroscopy. Their properties make them useful for various applications, including in chiral organic light-emitting diodes (CPL-OLEDs), catalysis, or nano-scale molecular machines.
Michael Pittelkow, University of Copenhagen, Denmark, and colleagues have developed a concise synthetic strategy for the synthesis of heterocyclic [9]helicenes that relies on oxidative cross-coupling reactions between 2-naphthols and hydroxy carbazoles (pictured below). The dihydroxy carbazole derivative that the team used can be prepared in five steps from carbazole. It is reacted with with two equivalents of the electron-rich 7-methoxy-2-naphthol. The following dehydration to yield the desired [9]helicene with two furans can be achieved by treatment with TsOH in boiling chlorobenzene.
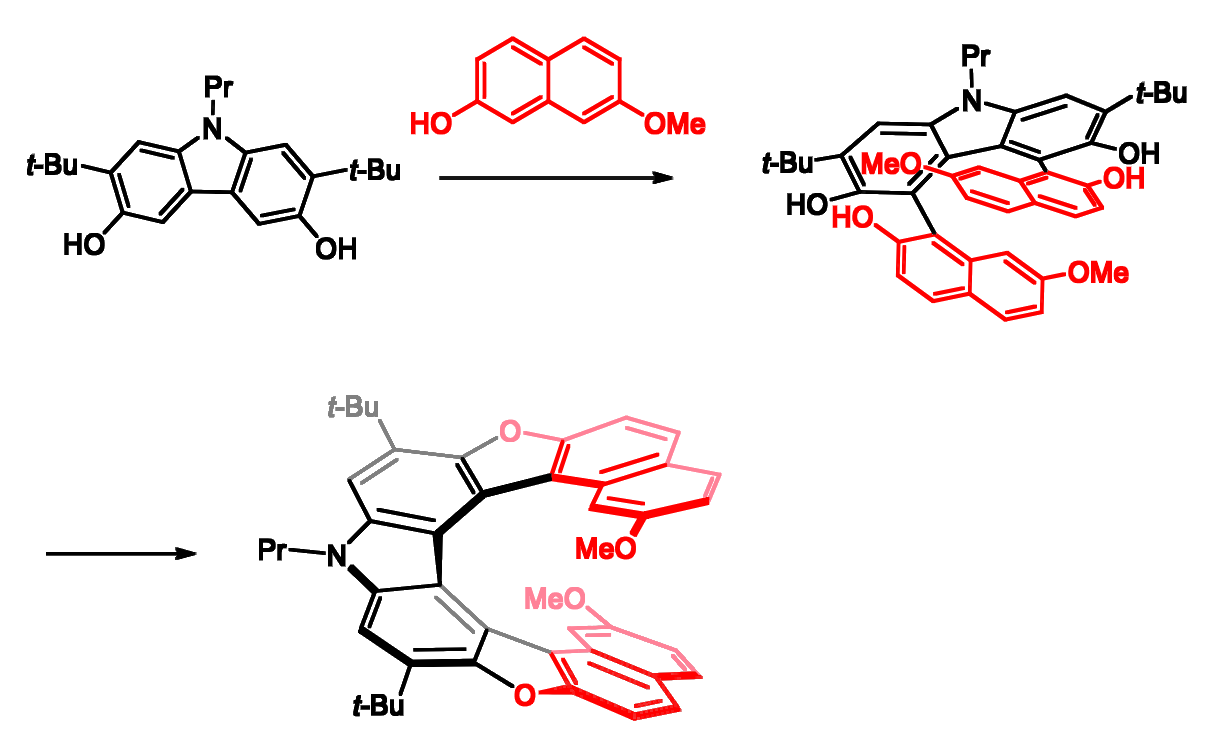
The team isolated the enantiomers of the helicene via demethylation of the methoxy groups followed by a reaction with (1R)-(–)-menthyl chloroformate as a chiral auxiliary. The resulting mixture of diastereoisomers was separated using standard chromatography, and the menthol carbonates were removed using LiAlH4 to obtain the enantiomers in high yields and purities.
The [9]helicenes displayed impressive CPL brightness in the blue region—the highest among their heterocyclic counterparts. The team also used quantum-chemical calculations to predict and understand the (chir)optical properties of the helicenes. This combination of experimental findings and quantum chemical calculations offers a comprehensive understanding of these molecules and could open up possibilities for their use in a range of applications.
- Heterocyclic [9]helicenes exhibiting bright circular polarized luminescence,
Stefan S. Warthegau, Andreas E. Hillers-Bendtsen, Stephan K. Pedersen, Cecilie Rindom, Christoffer Bræstrup, Jeppe S. Jensen, Ole Hammerich, Maria S. Thomsen, Fadhil S. Kamounah, Patrick Norman, Kurt V. Mikkelsen, Theis Brock-Nannestad, Michael Pittelkow,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202301815
![Concise Synthesis of Heterocyclic [9]Helicenes](https://www.chemistryviews.org/wp-content/uploads/2023/08/heterocyclic9helicenes_2023.png)



