In quantum tunneling, particles move, or “tunnel”, through an energy barrier and appear on the other side without moving over the barrier. This is impossible in classical mechanics, but happens with a certain probability for objects that are small enough to be governed by quantum mechanics.
Quantum tunneling can affect chemical reactions, most commonly when hydrogen atoms are involved. Usually, classical transition state theory is used to predict the outcomes of reactions, where reactions proceed by overcoming an energy barrier and not tunneling through it. However, tunneling may significantly influence the chemical reaction outcome in more cases than previously thought.
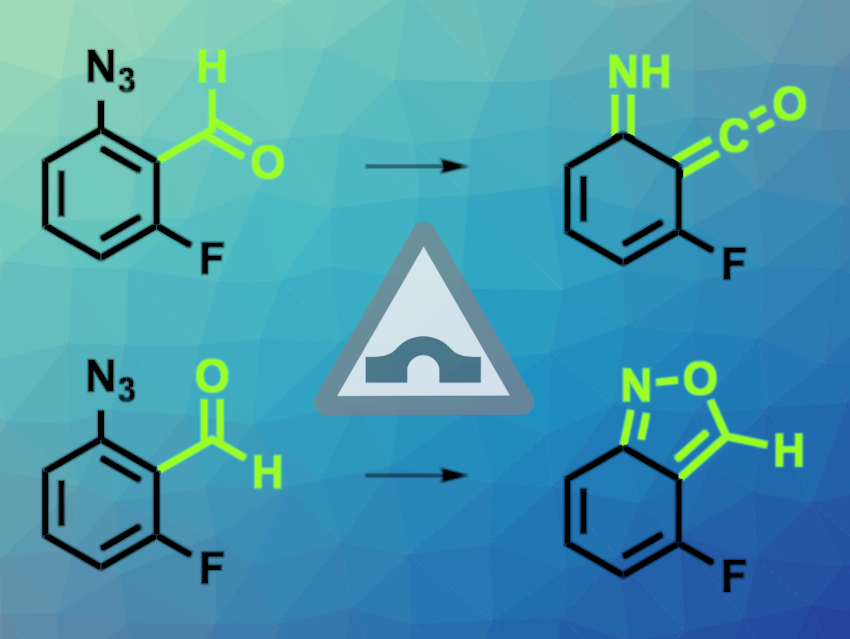
Cláudio M. Nunes, University of Coimbra, Portugal, and colleagues have found an unprecedented example where two conformers of the same species undergo tunneling reactions to distinct rearrangement products (pictured). The team generated anti– and syn-conformers of 2-formyl-3-fluorophenylnitrene in a nitrogen matrix at 10 K. The nitrenes were prepared from 2-formyl-3-fluorophenyl azide precursors (pictured above on the left), using light irradiation to cleave N2 from the azide groups. The researchers observed the subsequent spontaneous reactions using IR spectroscopy. Signals stemming from the two conformers can be told apart because they react at different speeds.
The team found that the anti-conformer rearranges to an imino-ketene (pictured above on the first line) with a half-life of ca. 2 min. This reaction involves hydrogen atom tunneling. The syn-conformer rearranges to a 2,1-benzisoxazole (pictured above on the second line) with a half-life of ca. 10 min. This slower reaction involves the tunneling of heavier atoms. Under the reaction conditions, no isomerization between the two conformers was observed. Both of the rearrangement reactions have even higher energy barriers than this isomerization, which shows that they must have occurred by tunneling and that the reactions cannot be explained by classical theories.
- Simultaneous Tunneling Control in Conformer-Specific Reactions,
Cláudio M. Nunes, José P. L. Roque, Srinivas Doddipatla, Samuel A. Wood, Robert J. McMahon, Rui Fausto,
J. Am. Chem. Soc. 2022, 144, 20866–20874.
https://doi.org/10.1021/jacs.2c09026



really marvelous and stunning research outcomes