As an emerging class of fluorescent molecules, molecular rotor-based fluorophores (RBFs) show fluorescence that depends on the rotation of a specific bond. This effect is sensitive to the local viscosity. In a low-viscosity environment, RBFs rotate and undergo a twisted intramolecular charge transfer (TICT) process, which quenches fluorescent emission. In a viscous environment, the TICT process is inhibited, resulting in enhanced fluorescence. RBFs can be used, e.g., for detecting protein conformational changes.
Modulating Rotational Barriers
The key to controlling the viscosity sensitivity of rotor-based fluorophores is changing the rotational barrier along the rotating group in the excited state. Strategies such as altering the π-electron density can be used for this. However, these methods are generally either limited to one specific class of RBFs or cause undesirable shifts in the emission spectra. Therefore, a strategy that could be applied to multiple RBF scaffolds without perturbing their spectra would be useful.
Yu Liu, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, He Huang, Nanjing Normal University, China, Xin Zhang, Westlake University and Westlake Laboratory of Life Sciences and Biomedicine, Hangzhou, China, and colleagues have used hydrogen bonds to regulate to viscosity sensitivity of RBFs. They found that intramolecular hydrogen bonds increase the rotational barriers, and thus, change the viscosity sensitivities of multiple RBF probes.
Visualizing Protein Aggregation
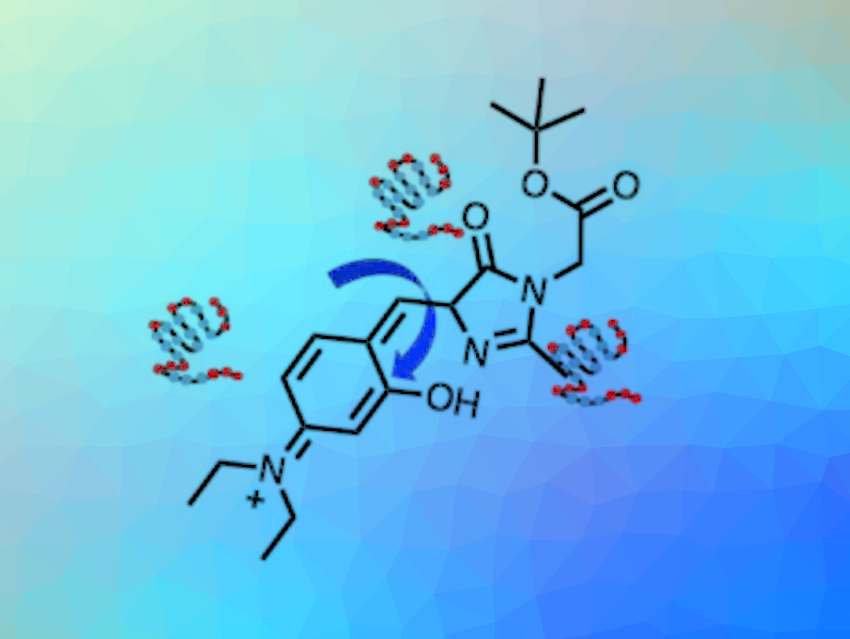
The team used 4-hydroxybenzylidene-imidazolinone (HBI)-based probes with intramolecular H-bonds to visualize a multi-step protein aggregation process. They installed groups that can form hydrogen bonds with different strengths (O−H···O, N−H···N, and O−H···N) in the probes, leading to three derivatives with different viscosity sensitivities. The derivative with the highest rotational barrier can activate fluorescence at the lowest viscosity. The local viscosity increases during the multi-step protein aggregation process, therefore, RBFs with different viscosity sensitivities and different fluorescence colors can be used to distinguish the aggregation states.
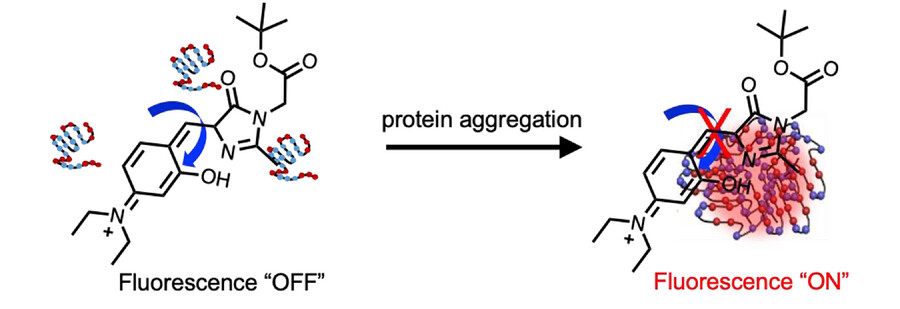
Overall, the work provides a new mechanism to rationally control the viscosity sensitivity of RBFs. The developed strategy could also be used together with other mechanisms. The ability to design fluorophores with tunable excited state rotational barriers could provide opportunities for new applications in biological and materials sciences.
- Installing hydrogen bonds as a general strategy to control viscosity sensitivity of molecular rotor fluorophores,
Baoxing Shen, Lihua Liu, Yubo Huang, Jichun Wu, Huan Feng, Yu Liu, He Huang, Xin Zhang,
Aggregate 2023.
https://doi.org/10.1002/agt2.421