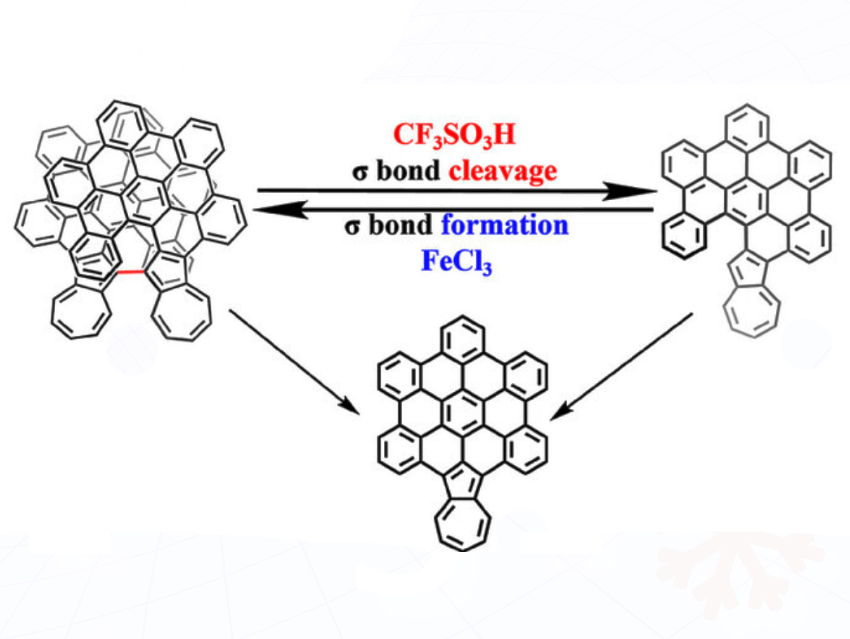
Precise control over nanographene aggregation is essential for elucidating its structure–property relationships. Conventional approaches that rely on non-covalent interactions, such as van der Waals forces and π–π stacking, often lack the resolution required for fine-tuned modulation. Han-Yuan Gong, Beijing Normal University, Beijing, China, and colleagues have reported a covalent regulation strategy based on reversible σ-bond formation and cleavage, enabling controllable switching between nanographene monomers and dimers with high efficiency.
The team has designed azulene-edged nanographene molecules whose aggregation state can be reversibly modulated by external stimuli. Protonic acid (CF₃SO₃H) induces σ-bond cleavage to generate monomers, whereas oxidative conditions using a Lewis acid (FeCl₃) promote σ-bond formation, regenerating dimers. By adjusting the reaction temperature and employing quenching agents such as H₂O or NEt₃, selective access to dimers or monomers was achieved; for instance, H₂O quenching at room temperature favors dimers, while NEt₃ yields monomers.
The resulting covalent dimer displays enhanced optoelectronic characteristics, including increased molar absorptivity, stronger fluorescence, extended electrochemical windows, and robust chiral stability. This covalent regulation strategy, in which azulene functions as a reactive site, overcomes the limitations of traditional non-covalent approaches. The researchers say the work not only provides a versatile platform for stimuli-responsive materials but also advances the development of aggregation-controlled functional nanographene systems.
- α-Helix-Driven Regulation of Aqueous Circularly Polarized Luminescence in Homopolypeptide Self-Assembly
Jinhui Jiang, Ziyue Ye, Siwei Zhang, Fulong Ma, Lin Lu, Zijie Qiu, Jianwei Sun, Yu Xiong, Zheng Zhao, Jacky W. Y. Lam, Ben Zhong Tang,
Aggregate 2025
https://doi.org/10.1002/agt2.70147