Aromaticity is an important concept in chemistry. Aromaticity leads to increased thermodynamic stability in cyclic species, whereas antiaromaticity causes instability. Thus, it is generally challenging to synthesize antiaromatic species.
The commonly known Hückel aromatics contain planar structures with 4n+2 π-electrons. In contrast, Craig and Möbius aromaticity describe molecules with 4n π-electrons. Möbius aromaticity is characterized by a 180° “twist” in the phases of the involved p-orbitals in a “Möbius strip” conformation. Craig aromatics feature a pπ–dπ interaction for the 180° phase shift and can retain planar structures. Examples of Craig aromatics have been synthesized.
When it comes to antiaromatics, both Hückel and Möbius antiaromatics have been realized, while Craig antiaromatics. which have 4n+2 π-electrons, had remained unknown.
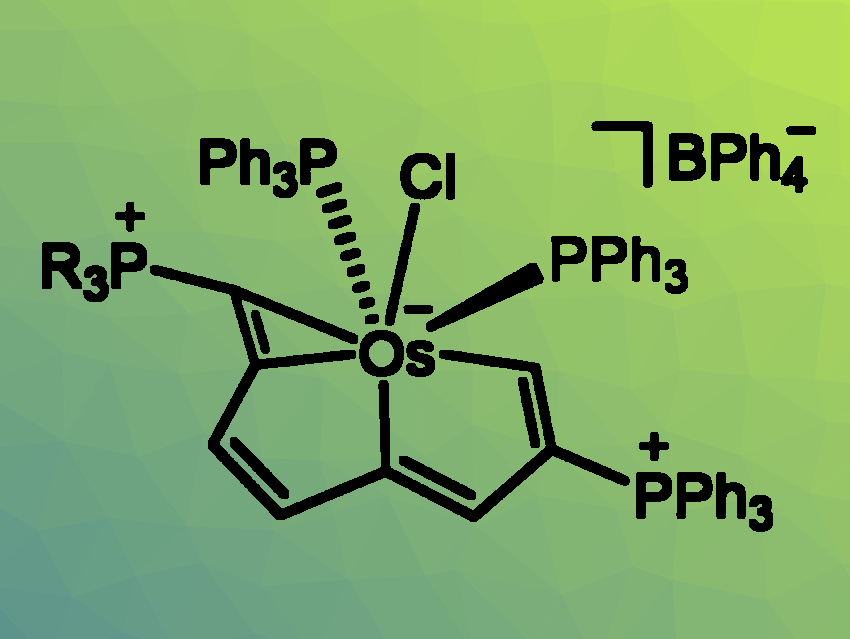
Jun Zhu, Xiamen University, China, Haiping Xia, Xiamen University and Southern University of Science and Technology, Shenzhen, China, and colleagues have reported the first synthesis of Craig antiaromatic species. Eight Craig antiaromatic compounds (example pictured) were synthesized via a deprotonation-induced reduction process, involving an acid-base neutralization and a redox reaction in one step. The team started by preparing different osmium complexes with two strongly electron-withdrawing phosphonium groups as precursors. The precursors were then reacted with Et2NH as a base to obtain the desired products.
Both experimental results and theoretical calculations confirmed the Craig antiaromaticity of the products. The researchers propose that it is the high exothermicity of the employed acid-base chemistry that drives this transformation.
- Synthesis and characterization of Craig-type antiaromatic species with [4n + 2] π electrons,
Lina Chen, Lu Lin, Amit Ranjan Nath, Qin Zhu, Zhixin Chen, Jingjing Wu, Hongjian Wang, Qian Li, Wen-Feng Lin, Jun Zhu, Haiping Xia,
Proc. Natl. Acad. Sci. USA 2023.
https://doi.org/10.1073/pnas.2215900120


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
