Boron-doped heterocycles can have interesting electronic or photophysical properties. Boraacenes are boron-doped polycyclic aromatic hydrocarbons (PAHs) that have potential applications, e.g., in organic electronics. Examples of boraacenes that are based on PAHs larger than anthracene, such as borapentacenes, are rare. (Pentacenes are compounds based on five linearly fused benzene rings.)
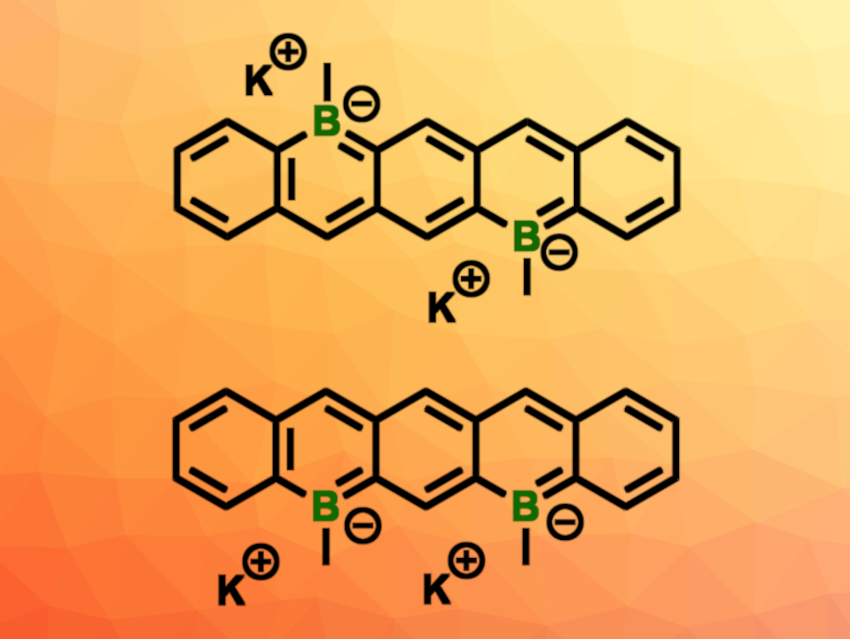
Robert J. Gilliard, Jr., University of Virginia, Charlottesville, USA, and colleagues have synthesized two dipotassium diboratapentacene isomers (pictured), i.e., 5,12- and 5,7-diboratapentacenes. The team started from 2,5-dibromoterepthalaldehyde or 2,4-dibromoisophthalaldehyde, which were connected to two equivalents of another arene building block using Grignard reactions. The resulting alcohols were reduced, followed by a conversion to tetramethyldisilapentacenes.
A silicon/boron exchange using BBr3 was then used to introduce the desired boron atoms. A methylation step and deprotonation with potassium bis(trimethylsilyl)amide (KHMDS) then gave the desired products, dipotassium 5,12-dimethyl-5,12-diboratapentacene (pictured above in the top row) and dipotassium 5,7-dimethyl-5,7-diboratapentacene (pictured above in the bottom row).
According to the researchers, the products are the first examples of fully aromatized diboraacenes with the boron atoms separated in different rings of the acene framework. The team found that both isomers react with CO2, resulting in a carboxylation of the pentacene backbone.
- Boron-Doped Pentacenes: Isolation of Crystalline 5,12- and 5,7-Diboratapentacene Dianions,
Joshua E. Barker, Akachukwu D. Obi, Diane A. Dickie, Robert J. Gilliard,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.2c11494