Cyclic arrays of porphyrin units could be useful, e.g, as model compounds for light-harvesting complexes. In contrast to supermolecular arrays, covalently bonded cyclic structures are better defined, but more challenging to prepare.
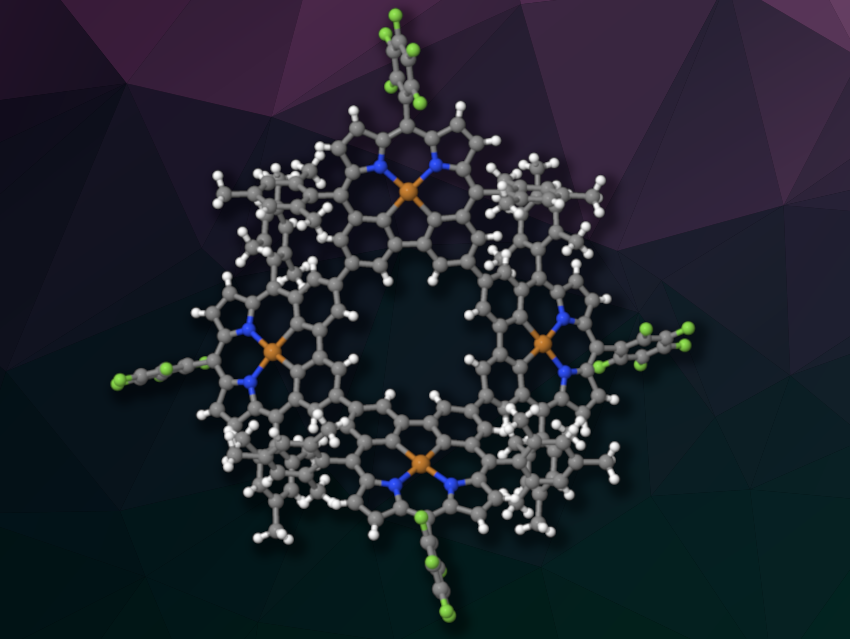
Dongho Kim, Yonsei University, Seoul, Republic of Korea, Jonathan L. Sessler, The University of Texas at Austin, USA, Xian-Sheng Ke, Beijing Normal University, China, and colleagues have prepared two cyclic porphyrin-like arrays, a trimer and a tetramer (latter pictured), based on carbaporphyrin. Carbaporphyrins are porphyrin analogues in which one or more nitrogen atoms are replaced by a carbon atom.
The team started from 3,3′,5,5′-tetrabromo-1,1′-biphenyl, which was reacted with mesitaldehyde to give a bicarbinol. A reaction with pyrrole then gave a dipyrrole intermediate. A condensation with pentafluorobenzaldehyde and an oxidation with 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) resulted in a dibrominated carbaporphyrin building block. This carbaporphyrin derivative was converted to its copper complex and then subjected to a nickel-catalyzed Yamamoto-type coupling.
The researchers obtained a cyclic trimer and a cyclic tetramer in yields of 14 % and 4 %, respectively. Single-crystal X-ray diffraction analysis showed that the trimer has a nearly planar structure, while the tetramer has a more saddle-like structure in which the carbaporphyrin units are distorted out of the plane. The team found evidence for excitation energy transfer (EET) within both the trimer and the tetramer, which is a key step in processes such as photosynthesis.
- Cyclic Carbaporphyrin Arrays,
Haodan He, Seokwon Lee, Ningchao Liu, Xiaotong Zhang, Yuying Wang, Vincent M. Lynch, Dongho Kim, Jonathan L. Sessler, Xian-Sheng Ke,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.2c11788