Oxygen-containing functional groups such as alcohols, aldehydes, and ketones are very common, and methods for their conversion into other functional groups are, thus, useful. Deoxygenative functionalizations are interesting in this context, but can be difficult to achieve. Electrochemical approaches could help to overcome this hurdle and reduce oxygen-containing groups.
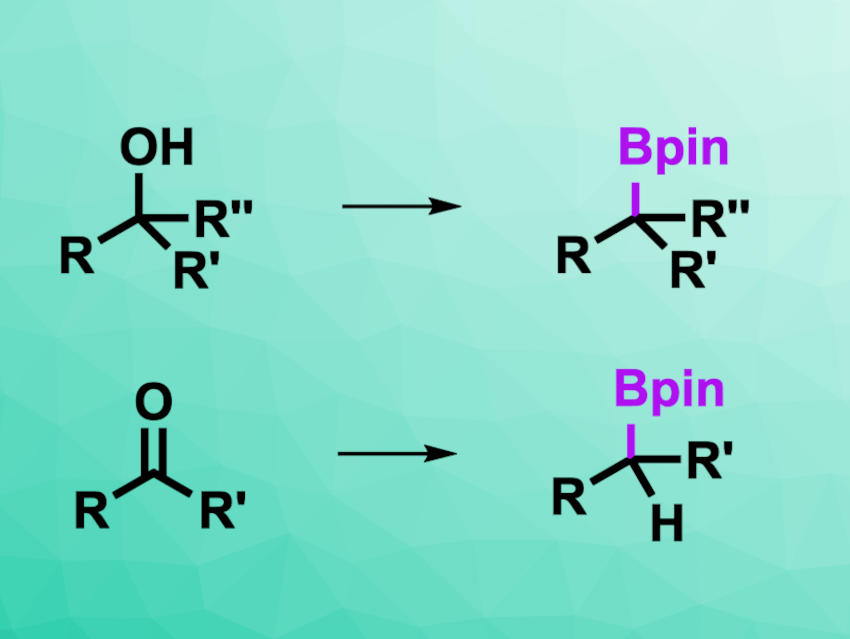
Song Lin, Cornell University, Ithaca, NY, USA, and colleagues have developed a method for the transformation of alcohols, aldehydes, and ketones into boronic esters using reductive electrochemistry (general reactions pictured). The team used pinacolborane (HBpin) as both an activator and a reactant together with a magnesium anode, a graphite cathode, tetrabutylammonium tetrafluoroborate as the electrolyte, and tetrahydrofuran (THF) as the solvent.
The desired products were obtained in moderate to high yields. The team was able to use the reaction for the borylation of natural products and pharmaceutically active compounds. The researchers propose that the substrate first reacts with pinacolborane as an activator to form a trialkylborate species, which can then undergo electrochemical reduction to a carbanion-type intermediate. This intermediate reacts with an additional equivalent of pinacolborane to give the desired borylated compound. The work could be useful, e.g., for the late-stage derivatization of complex structures that contain alcohols or carbonyl groups.
- Electrochemically Driven Deoxygenative Borylation of Alcohols and Carbonyl Compounds,
Weiyang Guan, Yejin Chang, Song Lin,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c03418


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
