Plastic waste is an environmental problem. Recycling can help, but mechanical recycling can lead to a decrease in the quality of the materials. Chemical recycling, in which polymers are converted to their monomers or other useful chemical feedstocks, can be a promising alternative. However, the chemical recycling of polymers with all-carbon backbones can be challenging due to their stability. Poly(methyl methacrylate) (PMMA) is one such polymer. It can be depolymerized to its monomer, methyl methacrylate (MMA), by pyrolysis, but this requires high temperatures of over 400 °C that can result in the formation of byproducts.
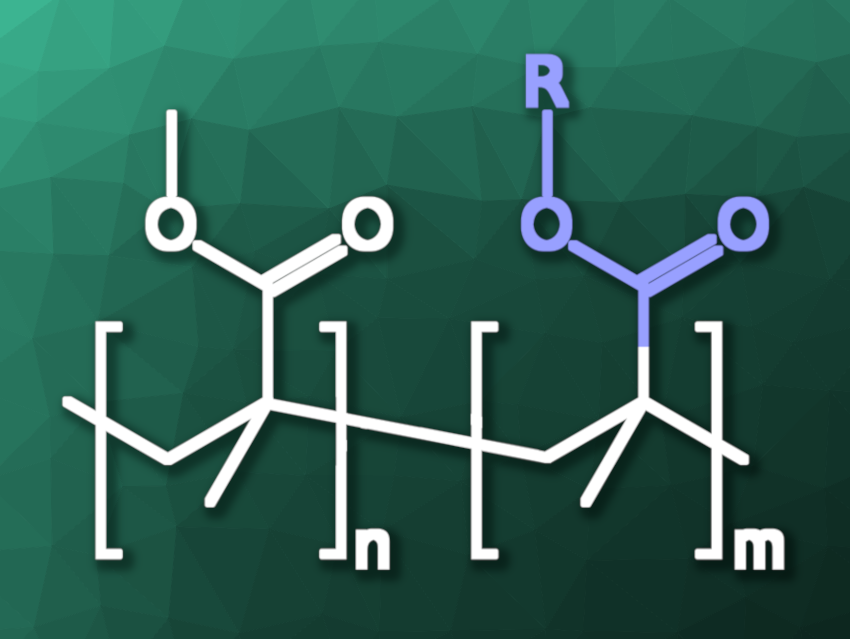
Brent S. Sumerlin, University of Florida, Gainesville, USA, and colleagues have developed depolymerizable PMMA derivatives by including thermolytically labile pendant groups at some of the monomers, which can be cleaved to generate backbone radicals and induce depolymerization. The team included 1–5 mol% of N-hydroxyphthalimide ester-containing monomers in addition to MMA to create the depolymerizable materials. They used monomers such as N-(methacryloxy)phthalimide (PhthMA), N-(methacryloxy)tetrachlorophthalimide (TCPhthMA), or N-(methacryloxy)succinimide (SMA) for this purpose.
The temperature required for depolymerization can be tuned by using these different monomers with thermolytically labile pendant groups and by varying their mass ratio in the polymer. The team found average depolymerization temperatures of 190 °C for TCPhthMA, 205 °C for PhthMA, and 232 °C for SMA. The developed approach allows for high degrees of depolymerization without the use of catalysts to generate MMA monomers. Thus, it provides a simple route to chemically recyclable PMMA derivatives.
- Bulk Depolymerization of Methacrylate Polymers via Pendent Group Activation,
Rhys W. Hughes, Megan E. Lott, Isabella S. Zastrow, James B. Young, Tanmoy Maity, Brent S. Sumerlin,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c14179