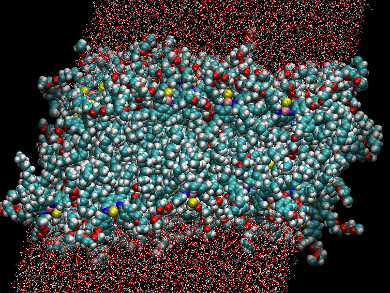
Vesicles formed from amphiphilic molecules can promote organic syntheses in aqueous media by forcing the substrates together into tight spaces. They can also be used to house catalysts for the reaction, allowing them to function as convenient nanoreactors. However, due to their size and fluidity, characterizing these nanostructures on a molecular level can be difficult.
Susumu Okazaki, Nagoya University, Japan, Yasuhiro Uozumi, Institute for Molecular Science, SOKENDAI, JST-CREST, and JST-ACCEL, Okazaki, Japan, as well as RIKEN Center for Sustainable Resource Science, Wako, Japan, and colleagues have elucidated the structure of a vesicle self-assembled from an amphiphilic palladium complex.
Molecular dynamics calculations and experimental wide-angle X-ray scattering techniques were used to analyze the bilayer structure. The results indicate a vesicle with a bilayer thickness of about 6 nm and a free-energy barrier to membrane water permeation of 12 kJ/mol. This suggests that water and organic molecules can easily pass through the bilayer.
A Miyaura-Micheal coupling reaction was performed in water using the palladium complex as a catalyst. The desired 3-phenylcyclohexanone was produced in 83 % yield using the self-assembled vesicular complex, compared to only 7 % using the non-self-assembled complex, demonstrating the dramatic advantage of using a vesicle.
- Detailed Structural Analysis of a Self-Assembled Vesicular Amphiphilic NCN-Pincer Palladium Complex by Using Wide-Angle X-Ray Scattering and Molecular Dynamics Calculations,
Go Hamasaka, Tsubasa Muto, Yoshimichi Andoh, Kazushi Fujimoto, Kenichi Kato, Masaki Takata, Susumu Okazaki, Yasuhiro Uozumi,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201603494