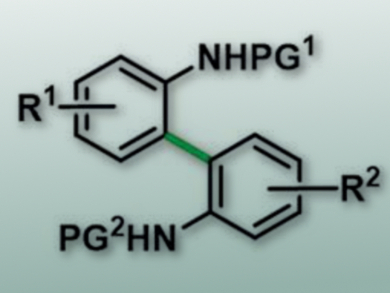
2,2′-Diaminobiaryls and their derivatives are common structural motifs, for example, in organic ligands and natural products. However, their synthesis can be difficult. Nonsymmetric products, in particular, require tedious synthetic protocols. A direct oxidative treatment of aniline and its derivatives usually results in the pitch-black electrically conductive polyaniline. This reaction takes place directly on anodes.
Siegfried R. Waldvogel, University of Mainz, Germany, and colleagues have developed a direct and selective electrochemical cross-coupling of aniline derivatives. This carbon-carbon bond formation requires no leaving groups, metal catalysts, or reagents. The team used a simple beaker with two electrodes (e.g., made from glassy carbon) and a constant current. The protective groups (PG) for the amino groups are common and simple (e.g., acetyl). The selectivity for the cross-coupling is based on a solvent effect of 1,1,1,3,3,3-hexafluoroisopropanol (HFIP), which modulates the nucleophilicity of the individual coupling components, and is the key for a clean electrochemical synthesis.
The electrochemical preparation shows good yields, and the experimental set-up is very simple. A broad scope of diaryldiamines (pictured) can be accessed by this method.
- Reagent- and Metal-Free Anodic C–C Cross-Coupling of Aniline Derivatives,
Lara Schulz, Mathias Enders, Bernd Elsler, Dieter Schollmeyer, Katrin M. Dyballa, Robert Franke, Siegfried R. Waldvogel,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201612613