Many natural products and drugs contain 3-aminosugars, which can be structurally modified to discover new drugs. However, the development of a general synthetic route to aminosugar-containing compounds is hampered by the structural diversity of aminosugars and the challenging nature of glycosidic bond construction.
Jing Zeng, Qian Wan, Huazhong University of Science and Technology, Hubei, China, and colleagues have developed a concise, diversity-oriented method for the synthesis of 3-aminosugars. A series of naturally occurring 3-amino- and 3-nitro-2,3,6-trideoxypyranoses and their analogues were obtained by functionalizing key intermediates derived from simple sugars. Further transformation of the aminosugars led to various glycosyl donors, which were coupled with glycosyl acceptors in a stepwise fashion by Yu glycosylation.
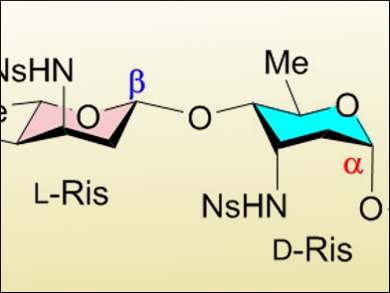
The researchers controlled α/β-linkage selectivity by modifying the glycosylation conditions: using dimethylformamide (DMF) as an additive reversed the selectivity. The team was able to selectively and efficiently form α- and β-linkages in a tetrasaccharide containing four different aminosugars (partially pictured above).
- 3-Aminodeoxypyranoses in Glycosylation: Diversity-Oriented Synthesis and Assembly in Oligosaccharides,
Jing Zeng, Guangfei Sun, Wang Yao, Yangbin Zhu, Ruobin Wang, Lei Cai, Ke Liu, Qian Zhang, Xue-Wei Liu, Qian Wan,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201700178