All plants produce considerable amounts of polyphenols as secondary metabolites, which then serve as flower pigments, protection from UV light, and defense against the plant’s being eaten. Polyphenols are regarded as healthy substances since they are thought to shield people from “illnesses of civilization” such as cardiovascular disease, cancer, or diabetes. But what specific compounds show a prophylactic effect with respect to these conditions, and how do they work?
1. What Are Polyphenols? Where Do They Come From?
At least from the standpoint of name, polyphenols constitute one of the less familiar natural-product classes, compared, for example, with proteins, fats, nucleic acids, sugars, or even alkaloids. Nevertheless, as contributors to good health, polyphenols are indeed familiar to almost everyone – under the broader heading “antioxidants.”
From a quantitative standpoint, polyphenols are among nature’s most important compound categories. With an overall roughly 30 % share of the world’s total biomass, polyphenols are second only to sugars as the most abundant organic compounds. Plants are the source of most of the polyphenols. Quantitatively, the most important representatives are wood (chemically: lignin, a copolymer of sugars and polyphenols) and humic acid, the decomposition product of dead plant material. In addition, all plants produce a variety of smaller polyphenols, with molecular weights under 1,000. To date, ca. 6,000 different natural compounds of this type have been identified.
Human beings, as omnivores, consume roughly 10 g of polyphenols per day from plant sources. It has been known for centuries that a high dietary content of plant matter – especially fruits and vegetables – is healthy. In the last two decades, this traditional knowledge, long passed down orally, has been closely examined scientifically, with the result that recent reductions in the incidence of cardiovascular disease, cancer, osteoporosis, and diabetes are thought to be due to consumption of larger amounts of fruits and vegetables. The immediate source of the positive health effects, apart from vitamins and minerals, is widely thought to be polyphenols.
2. Polyphenols: A Definition
For our purposes, a compound with a molecular weight above 600 Da and containing more than two phenol rings can be classed as a polyphenol. Substances with only one or two such rings are more correctly referred to simply as phenols, although these, too, are usually included (incorrectly) among the “polyphenols” [1].
 |
|
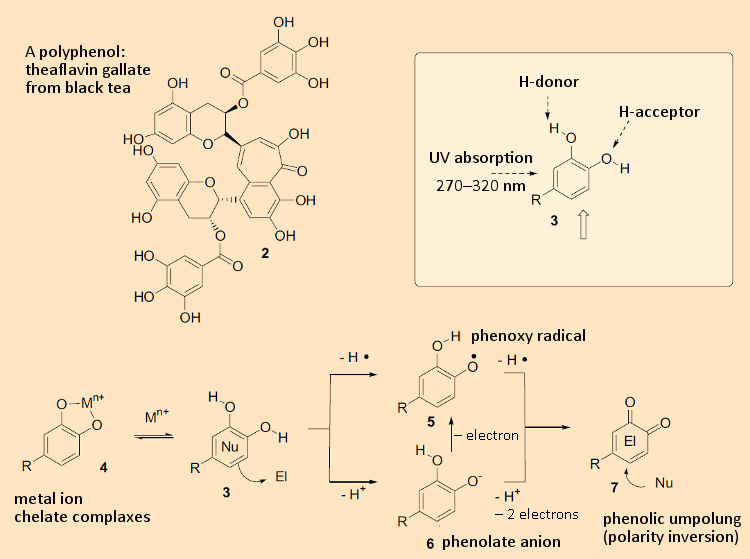
Figure 1. Phenols: structures and reactivity. |
3. Why Do Plants Make Polyphenols?
Without exception, all plants produce polyphenols, and in almost all of their various parts. The largest quantities are found in leaves and fruits. The primary role of polyphenols within the plants themselves relates to their ability to absorb UV light and to protect the plants from this radiation [1,2].
In general, one distinguishes between two classes of these compounds, based on their ability to protect against UV rays: flavonoids, which absorb in the region 220–300 nm and provide protection against so-called UV-A rays, and hydroxycinnamic acids, absorbing at 300–350 nm and offering protection against UV-B light.
Based on genetic studies, it is assumed that plant life on earth, upon leaving the waters of the oceans and settling on land, experienced a dramatic need to acquire UV protection. This evolutionary pressure led to the ability to form polyphenols. Animals, including humans, in the course of their own evolution, largely lost the genetic information necessary for polyphenol biosynthesis. The reason for this was the development of a skin as protection against UV radiation. Despite everything, however, we still continue to make use of the same basic ability of phenols to absorb UV light, because the protective skin pigment melanin is itself an oxidation product of the phenolic amino acid tyrosine.
Through evolution, plants have come to take advantage of, and optimized, a wide range of other characteristics of polyphenols. These compounds help protect plants from being eaten, both by microorganisms like bacteria and viruses and by larger herbivores. This is due, among other things, to the compounds’ unpleasant taste (more on that later). Polyphenols also serve as coloring matter both in flowers and also more generally, and they can prove attractive to certain insects (see 5-caffeoylquinic acid (10) in Figure 2).
|
|
|
Figure 2. Chemical structures of hydroxycinnamic acid derivatives (8–13) and flavonoids (14–19). |
4. Chemical Properties
By definition, polyphenols are compounds with multiple aromatic rings bearing multiple OH-groups. Due to its free electron pair, even a single OH-group makes an aromatic ring electron-rich, so all polyphenols (3) in general are very good nucleophiles, and are readily oxidized. In providing electrons, they behave reductively, or – expressed differently – serve as antioxidants (see Fig. 1). The latter is one reason why they are utilized in foodstuffs.
Polyphenols can form chelate complexes (4) with metal ions [1,2]. The hydrogen atom of the phenolic OH-group is responsible for the special chemical characteristics of this class of substances. It can be easily removed chemically in two ways: in the context of an acid-base reaction, or as a free radical. Phenols display a lower pKa value (i.e., are more acidic) relative to alcohols like ethanol, which is to say this proton can be removed quite easily with a base. The reaction is facilitated by the phenol’s overall chemical structure: The resulting negative charge is stabilized with the aid of the aromatic ring through resonance (phenolate anion, 6). If the hydrogen is instead removed as a radical, there remains a free, unpaired electron on the phenol oxygen (see 5). Here, just as with the acid-base reaction, resonance stabilization plays an important role for the radical. Due to the radical’s resulting stability, a phenol also becomes a good reaction partner for other radicals.
The latter characteristic is the more pronounced the more OH-groups are present on an aromatic ring. Both the phenoxy radical (5) and the phenolate anion (6) can be oxidized in subsequent oxidation steps to an ortho-quinone (7). The latter is a reactive electrophile that can react with nucleophilic cellular components. Thus, oxidation makes it possible for the chemical reactivity of a phenol to be transformed from that of a nucleophile to an electrophile, thereby accomplishing a polarity inversion or “umpolung”. This, in turn, leads to phenols reacting with one another and, through the creation of new C–C bonds, oligomerization and polymerization reactions, which are especially important in the formation of lignin.
All phenols have the further possibility, through their OH-groups, of entering into hydrogen bonding with other biological molecules. Their electron-rich aromatic rings facilitate interactions particularly with cations and with other aromatic rings. As a consequence, polyphenols are found to interact above all with proteins. Because of this “binding” property, such species might be described also as natural “adhesives” for proteins.
Their chemical nature – above all the high reactivity and a tendency especially toward interaction with other substance classes – complicates experimental work with polyphenols. Natural product chemists regard them as extremely difficult, and as unrewarding with respect to scientific productivity. This may be one reason why today very few chemical research groups work with polyphenols, despite their great relevance.
5. Substance Classes
Polyphenols can be classified in many ways. Thus, they can be sorted according to function (UV-A and UV-B protection), or based on chemical structure (large or small, number of aromatic rings, etc.).
Hydroxycinnamic acids, Flavonoids, and Tannins
For a chemist, the most natural approach is classification based on chemical properties and fundamental structure, which is, in turn, a function of their biosynthesis. Thus, such phenols are divided into hydroxycinnamic acids (with 9 carbon atoms), flavonoids (with 15 carbon atoms), and tannins (with many carbon atoms, see Fig. 3).
 |
|
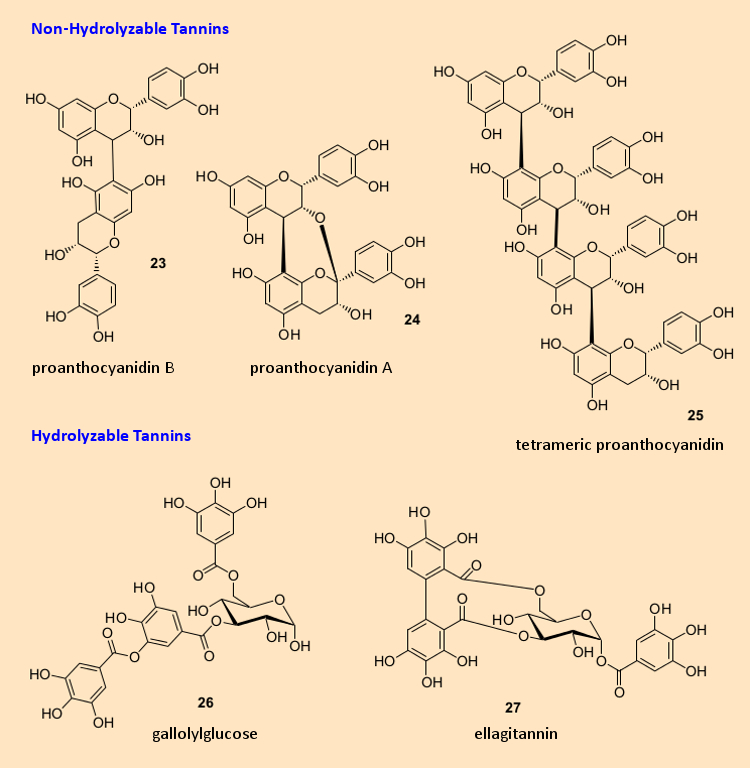
Figure 3. Chemical structures of tannins (21–27). |
These are further divided into subclasses: among the hydroxycinnamic acids, there are free acids such as caffeic acid (8) (the trivial name typically reveals a compound’s origin), or ferulic acid (9), or esters of caffeic acid like chlorogenic acid (10), cynarin (11), or chicoric acid (12), as well as other coupling products like rosmarinic acid (13).
Among the flavonoids, one makes distinctions on the basis of the oxidation level of the unsaturated pyran ring. There are flavonols (with OH-groups) such as epicatechin (14) or epigallocatechin gallate (15)), flavanones (with C=O-groups) such as luteolin (16), flavanolones like quercetin (17), open-chain derivatives such as naringenin (18), or isomeric isoflavones like genistein (19).
Important compounds like stilbene resveratrol (20) are not readily incorporated into any of these categories, however. The tannins (see Fig. 3) are subdivided into hydrolyzable tannins like (21) and (22) (these can be cleaved by bases into smaller molecules) and non-hydrolyzable tannins like (23) – (25), which are derived from flavanols (14) by oxidation. The nomenclature is confusing and to some extent chaotic, since it developed historically and was unfortunately never systematized.
6. Analysis and Structure Determination
As with other natural products, the job of structure determination always begins with a chemical separation. This normally entails the use of liquid chromatography with the aid of various column materials. After isolation of a pure compound starting from plant material, a structure is established with the aid of modern spectroscopic methods, such as NMR, infrared (IR), and UV/Vis spectroscopy and mass spectrometry (MS).
As already noted, polyphenols are difficult compounds to manage. To begin with, they are very reactive, so that at each analytical step one must make provisions to prevent their decomposition. Furthermore, the plants themselves contain a great many compounds (typically around 50). The record-holder in this regard is the coffee plant (Caffea conifera), which itself may generate 120 different phenolic chlorogenic acids (isomers of (10) and (11)) [3]. Such diversity not only complicates the isolation and purification of individual substances, but also creates immense challenges with respect to structural clarification.
In recent years, mass spectrometry (MS) has shown itself to be ideal for characterizing polyphenols. Of all the available analytical methods, MS offers the highest resolution; in a single experiment, a great many compounds can be detected simultaneously, and with high sensitivity. Moreover, mass spectrometry is beautifully suited to direct coupling with chromatographic separation techniques (LC-MS methods). Thus, in a single LC-MS experiment, hundreds of compounds can be separated and have their molecular formulas established, and subsequently, have structures assigned through fragmentation experiments and with the aid of the resulting spectra. Today, there even exist reliable MS methods for distinguishing among isomeric compounds [4].
7. Polyphenols in Everyday Life
Due to their chemical structures and the resulting properties, polyphenols have acquired a series of interesting industrial applications. For example, the fact that they bind firmly to proteins, and thus denature them (i.e., cause them to become insoluble), is important in the making of leather. An animal skin is first treated with phenolic tanning agents, causing the phenols to bind to proteins in the hide, which precipitate, and leather is formed. A similar protein precipitation is also exploited in beer production: Addition of polyphenols to naturally turbid beer causes proteins to be denatured and precipitated out. Subsequently, these proteins can readily be removed by filtration [1].
Astringent and Bitter Substances
Polyphenols are even capable of precipitating proteins on the human tongue, especially the so-called salivary proteins. Precipitation of these salivary proteins is perceived as astringency (lat. adstringere = contract), i.e., the formation of a rough, furry mouthfeel.
Many polyphenols also interact with bitter-receptors on the tongue. Plants probably once relied upon this effect to discourage herbivores from consuming phenol-rich plant components. Many people, however – and animals as well – become accustomed to astringent and bitter flavors, and indeed learn to cherish them.
Pigments
Phenols in the anthocyanin category often are colored, and function in plants as coloring agents in blossoms [5], or as pigments in other plant parts – e.g., in the fruits of red, blue, and black currants, blueberries, blackberries, raspberries, or cranberries, They also provide the red in beets and red cabbages, and the colors in apple peel.
 |
|
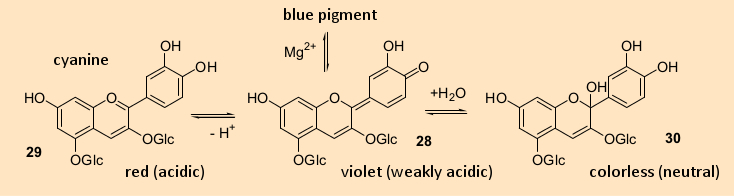
Figure 4. Structures and color changes of anthocyanins as plant pigments. |
Some polyphenols change their colors as a function of the pH of their environment, like litmus and other pH indicators (see Fig. 4). Many such color changes occur through complexation of anthocyanins with metal ions, including magnesium, aluminum, potassium, or iron, as well as through co-pigmentation. In the latter situation, an anthocyanin dye attaches to another polyphenol, such as a flavonoid. With many flowers, a small change in pH, accumulation of metal ions, or co-pigmentation within the blossom can produce multiple colors simultaneously. A dual-colored pansy utilizes only a single pigment but provides different pH values in different parts of the blossom. Both a red rose and a blue cornflower (bluebottle, bachelor’s button) contain the same cyanine dye (28), but the blue color in a cornflower is a result of complexation with magnesium.
A similar phenomenon explains how it is that many flowering plants reveal the pH of the soil in which they are planted. This is especially dramatic with hydrangeas. If you were to buy a blue hydrangea and plant it in weakly acidic (pH 6) soil, you could expect red blossoms to form in the subsequent year.
References
[1] S. Quideau et.al., Plant Polyphenols: Chemical Properties, Biological Activities and Synthesis, Angew. Chem. Int. Ed. 2011, 50, 586–621. https://doi.org/10.1002/anie.201000044
[2] O. M. Anderson, K. R. Markham (Eds.), Flavonoids – Chemistry, Biochemistry and Applications, CRC Press, 2005. ISBN: 978-0-8493-2021-7
[3] N. Kuhnert et al., Scope and limitations of principal component analysis of high resolution LC-MS data: The analysis of the chlorogenic acid fraction in green coffee beans as a case study, Anal. Meth. 2011, 3, 144–155. https://doi.org/10.1039/c0ay00512f
[4] M. N. Clifford et al., Hierarchical scheme for the LC–MS identification of chlorogenic acids, J. Agr. Food. Chem. 2003, 51, 2900–2911. https://doi.org/10.1021/jf026187q
[5] T. Goto, T. Kondo, Structure and molecular stacking of anthocyanins – Flower color variation, Angew. Chem. Int. Ed. 1991, 30, 17–33. https://doi.org/10.1002/anie.199100171
The article has been published in German as:
- Polyphenole: Vielseitige Pflanzeninhaltsstoffe,
Nikolai Kuhnert,
Chem. unserer Zeit 2013, 47, 80–91.
DOI: 10.1002/ciuz.201300589
and was translated by W. E. Russey.
Polyphenols: Contributors to Good Health – Part 1
What are polyphenols and why do plants make them?
Polyphenols: Contributors to Good Health – Part 2
How do polyphenols influence human health?
.jpg)


