Vinyldiazo compounds and vinylazides show a rich chemistry and have many applications in organic synthesis. In general, in the presence of a transition metal, both types of reagents undergo a facile nitrogen extrusion together with the generation of a highly reactive species.
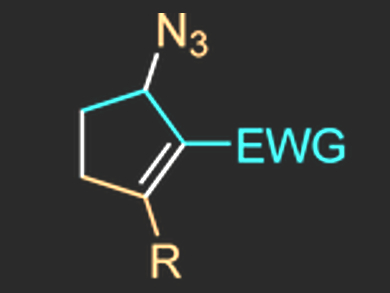
Enol López and Luis A. López, Universidad de Oviedo, Spain, have found suitable conditions for a copper(I)-promoted selective cyclization reaction of stabilized vinyldiazo compounds with vinylazides, despite the presence of multiple reactive sites in both reagents. In particular, the use of a readily available copper complex enabled the synthesis of cyclopentene derivatives while retaining the azide group (product pictured). The process involves an initial cyclization reaction and a subsequent migration of the azide group.
The reaction has a broad substrate scope and proceeds in good to excellent yields. The presence of the azide function in the final products provides a variety of synthetic opportunities.
- Synthesis of Functionalized Cyclopentene Derivatives from Vinyldiazo Compounds and Vinylazides through Sequential Copper-Promoted [3+2] Cycloaddition/Azide Rearrangement,
Enol López, Luis A. López,
Angew. Chem. Int. Ed. 2017, 56, 5121–5124.
DOI: 10.1002/anie.201701572