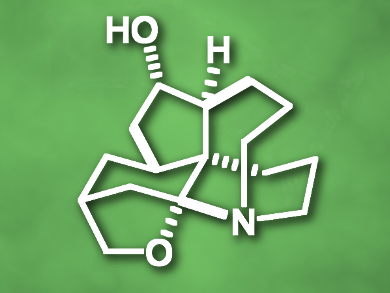
(–)-Huperzine Q, (+)-lycopladine B, (+)-lycopladine C, and (–)-lycopladine D are four structurally related fawcettimine-type lycopodium alkaloids, which are a large family of complex natural products with intricate polycyclic skeletons and diverse bioactivities.
Xiaoguang Lei, Peking University, China, and colleagues have developed a ten-step total synthesis of (–)-huperzine Q (pictured), total syntheses of (+)-lycopladines B (12 steps) and C (13 steps), as well as (–)-4-epi-lycopladine D (13 steps). The team used a unified strategy based on a series of cascade reactions. Starting from an enantiopure cyclohexanone derivative, a 6/9 spirocyclic ring was constructed via a Michael addition/aldol/intramolecular C-alkylation sequence, followed by an ethylene-accelerated carbonyl–olefin metathesis to form a 6/5/9 tricyclic skeleton.
Finally, a C4-epimerization/cyclization and late-stage enamine bromofunctionalization provides access to (–)-huperzine Q, (+)-lycopladine B, and (+)-lycopladine C, while a tandem C4-epimerization/retro-Claisen condensation gives (–)-4-epi-lycopladine D.
- Divergent Total Syntheses of (–)-Huperzine Q, (+)-Lycopladine B, (+)-Lycopladine C and (–)-4-epi-Lycopladine D,
Xiaoguang Lei, Benke Hong, Dachao Hu, Jinbao Wu, Jing Zhang, Houhua Li,
Chem. Asian J. 2017.
DOI: 10.1002/asia.201700364