Organoboranes are widely used in synthetic organic chemistry. The development of efficient and chemoselective methods for borylation reactions is an interesting research target.
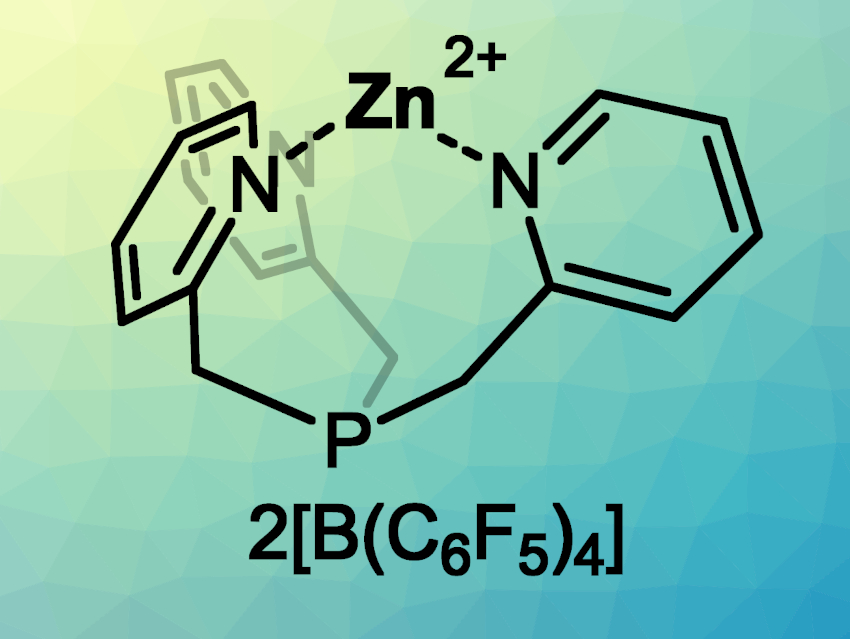
Roman Dobrovetsky, Tel Aviv University, Israel, and colleagues have synthesized an air-stable, dicationic, zinc complex using a tripod-type ligand (pictured) that features a phosphorus center and three pyridinyl “arms”. This complex was prepared by mixing ZnCl2 with tris(2-methyl-6-pyridylmethyl) phosphine) (TPPh), followed by the abstraction of two chlorides using K[B(C6F5)4].
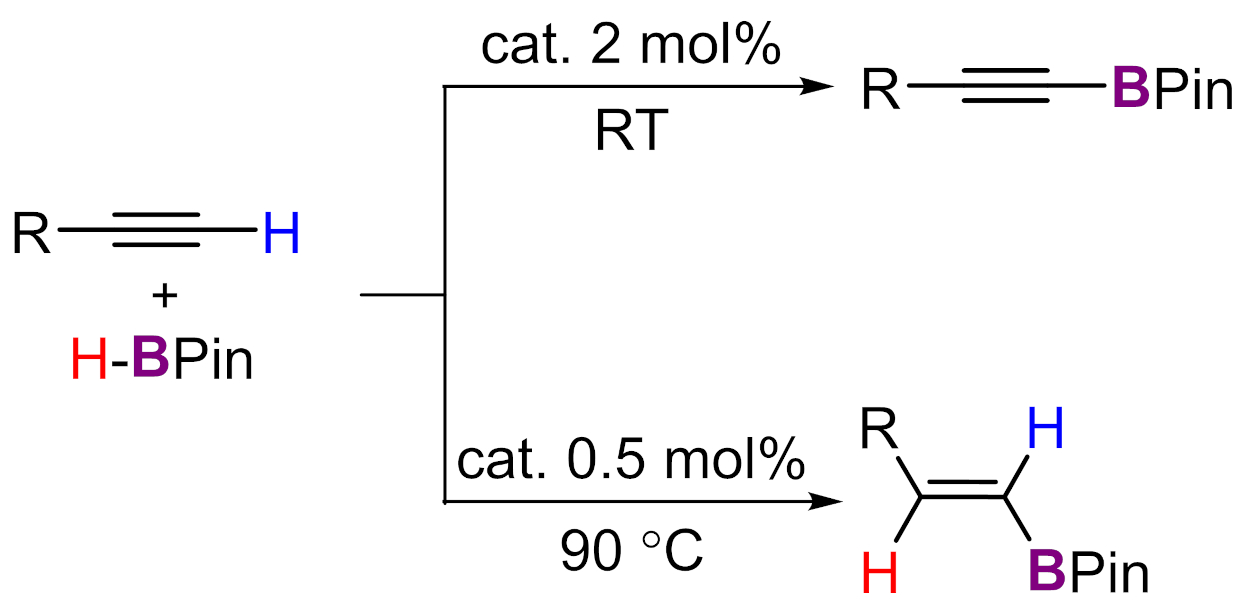
The resulting complex can catalyze two different borylation reactions of terminal alkynes depending on the reaction conditions (pictured below, HBPin = pinacolborane). Using 2 mol% of the catalyst at room temperature selectively catalyzes the dehydrogenative borylation of terminal alkynes. In contrast, 0.5 mol% of the catalyst at 90 °C selectively promotes a hydroboration reaction of alkynes. The researchers propose that the unchanged complex acts as the precatalyst for the dehydrogenative borylation, while at 90 °C, borenium-type catalyst species are formed from the complex and HBPin.
- An “On‐Demand”, Selective Dehydrogenative Borylation or Hydroboration of Terminal Alkynes Using Zn2+‐based Catalyst,
Kuldeep Jaiswal, Kristina Groutchik, Deependra Bawari, Roman Dobrovetsky,
ChemCatChem 2022.
https://doi.org/10.1002/cctc.202200004