Do Oil and Water Really Not Mix?
It is deceived wisdom that oil and water do not mix because on the microscale, they most certainly combine physically in ways that make the environmental cleanup of oil spills, the remediation of industrial sites, other environmental concerns a real problem. Taking inspiration from nature, Zhichao Dong, Chinese Academy of Sciences, Beijing, China, and colleagues have developed what amounts to a “go-in-opposite ways” separation of the water from the oil in microscopic droplets. Their approach, which requires no energy input, mimics the curved peristome (the structure around the opening) of the “pitcher” of the plant species Nepenthes alata, a tropical pitcher plant.
The researchers explain how separating water from oil in microdroplets is important environmentally in practical terms as well as in the laboratory, where it is critical in bioassays and other experiments. There are several approaches to the bulk separation of oil and water based on membrane and sponge technology. Separating mixtures accounts for 10 to 15 % of the world’s energy consumption, the team points out.
There have been many advances based on wettable and superwettable materials, as well as two-faced substances that display Janus wettability. Previous research used sol-gel systems, aerogels, polymer textiles, surface modifications, and hydrogel-coated meshes with micro- and nano-structures for separation in plug-and-go approaches. These methods each have their limitations and can require large amounts of energy to operate. However, the team’s observations of the properties of the pitcher plant suggested to them an alternative approach.
Mimicking the Pitcher Plant
Along the opening of the pitcher plant capsule, there is a peristome, a fringe of small overlapping projections. These tiny projections cause water condensing on the surface to spread in a single direction, making a very slippery surface. No energy input is needed to create this surface. The plant exploits this as insects, specifically ants seeking a free meal from the plant in the form of sweet nectar, quickly lose their footing and fall into the pitchers. There is no escape—once trapped within, they are digested and provide a useful nutritional source for the plant.
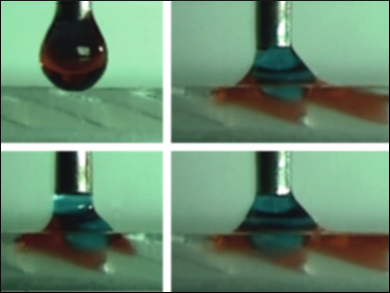
The team reasoned that if they could mimic the formation of this slippery surface, they might be able to apply water-oil microdroplets in which the water moves passively in one direction, separating from the oil. The team used a 3D printer to fabricate two artificial pitcher plant peristomes from either PDMS (polydimethylsiloxane) or PVA (polyvinyl alcohol). They provided a proof of principle by placing these two surfaces facing close to each other and adding water-in-oil microdroplets. The water from the droplets moves in one direction along one surface, while the oil passes in the other direction on the facing surface (pictured).
“Our device can spontaneously separate several microliter-sized water-in-oil droplets into pure oil and pure water droplets with controllable volumes ranging from nanoliters to microliters in controlled speeds, even within milliseconds,” the team told ChemViews Magazine. They point out that it is possible to adjust the 3D printing resolution from 20 to 200 µm, which means that they can fabricate a device for delicate separations of the kind used in bioassays and laboratory work or for ink separation in the printing industry. The team could also carry out a high-speed construction of a much larger apparatus with a lower resolution, which could be used for bulk separations in environmental cleanup.
- Peristome-Mimetic Curved Surface for Spontaneous and Directional Separation of Micro Water-in-Oil Drop,
Chuxin Li, Lei Wu, Cunlong Yu, Zhichao Dong, Lei Jiang,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201706665