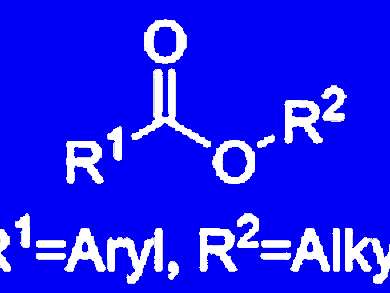
Functionalized alcohols are of importance for the manufacture of pharmaceuticals, agrochemicals, dyes, and numerous bioactive compounds. The development of a cost-effective, efficient, and highly selective catalyst for this transformation is still desirable because most of the known protocols either require expensive silanes or have limited functional group tolerance.
The abundant availability, low toxicity and biomimetic nature of zinc makes it a highly attractive candidate for catalysis.
Matthias Beller and his team, Leibniz-Institut für Katalyse e.V., University of Rostock, germany, have developed a general and chemoselective catalytic reduction of esters to alcohols using inexpensive zinc acetate and silanes. The operational simplicity and the high functional group tolerance, without the need for protecting and deprotecting steps, make this procedure particularly attractive for organic synthesis.
- Zinc-Catalyzed Chemoselective Reduction of Esters to Alcohols,
Shoubhik Das, Konstanze Möller, Kathrin Junge Matthias Beller,
Chem. Eur. J. 2011.
DOI: 10.1002/chem.201100800


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
