Many biologically active natural products, such as sugars and antibiotics, contain α-hydroxy carbonyl units. These have been abundantly used as a versatile chiral building block for the asymmetric synthesis of more complex molecular architectures. However, efficient methods for the preparation of chiral acyclic tertiary α-hydroxy carbonyl species are lacking. This is mainly due to the difficulty to prepare stereodefined polysubstituted enolates or silyl ketone aminals possessing two different alkyl groups on the sp2 carbon center.
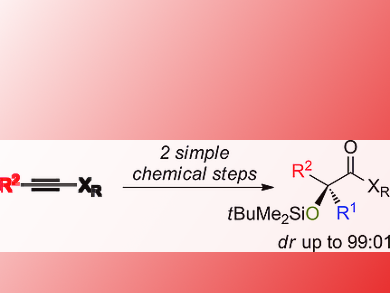
Jian Qiang Huang, Zackaria Nairoukh, and Ilan Marek, Technion-Israel Institute of Technology, Haifa, have developed a practical approach to acyclic tertiary α-hydroxy carbonyl compounds with excellent diastereoselectivity in only two chemical steps. The synthesis starts with a combined carbometalation-oxidation-silylation of alkynes. Stereodefined polysubstituted silyl ketone aminals are formed. The second step is an electrophilic oxidation reaction to acyclic tertiary α-oxidized carbonyl compounds possessing two different alkyl groups.
The products could be smoothly converted to synthetically useful enantiomerically enriched 1,2-diol derivatives.
- Electrophilic Oxidation of Stereodefined Polysubstituted Silyl Ketone Aminals,
Jian Qiang Huang, Zackaria Nairoukh, Ilan Marek,
Eur. J. Org. Chem. 2017.
https://doi.org/10.1002/ejoc.201701516