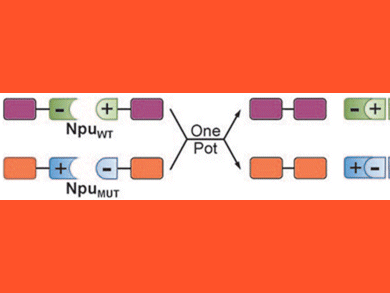
A new one-pot trans-splicing system based on the Npu split intein and a designed mutant of the same split intein has been described by Tom Muir’s team from the Rockefeller University (USA). This general ligation technique for peptides and proteins allows ligations of more than two pieces in a single reaction batch, which is rare. The authors uniquely exploit electrostatic principles to carry out charge-swapping of key intermolecular ion clusters in the naturally split DnaE intein from Nostoc punctiforme (NpuWT) alters split intein binding affinities and trans-splicing kinetics. This concept was used to engineer the new split intein NpuMUT with low cross-reactivity to NpuWT fragments. This intein pair was used to catalyze multiple trans-splicing reactions in one pot with kinetically controlled selectivity (see figure). In this was they managed to assemble the human poly(ADP-ribose) polymerase 1, which is an enzyme of high biomedical relevance and cannot be produced recombinantly in full-length. As noted by one referee “This concept could be the basis for other protein engineering and design approaches”.
- Kinetic Control of One-Pot Trans-Splicing Reactions by Using a Wild-Type and Designed Split Intein,
Neel H. Shah, Miquel Vila-Perelló, Tom W. Muir
Angew. Chem. 2011.
DOI: 10.1002/ange.201102909 - Angew. Chem. Int. Ed. 2011.
DOI: 10.1002/anie.201102909