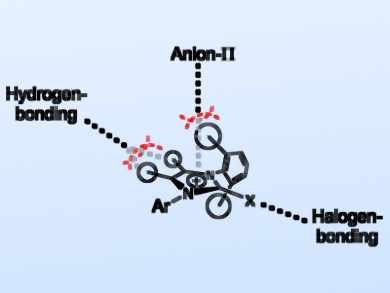
The rational design of new organocatalysts and anion receptors benefits from a deeper understanding of their binding modes in solution. Such structures may have multiple functionalities that can take part in different, potentially competing interactions. Thus, it is important to know which of these interactions dominate in solution.
Elsa Sanchez-Garcìa, Universität Duisburg-Essen and Max-Planck-Institut für Kohlenforschung, Mülheim an der Ruhr, both Germany, Stefan M. Huber, Ruhr-University Bochum, Germany, and colleagues have studied the non-covalent interaction modes of iodoimidazolium salts with anions in organic solvents. Such systems may interact with halide anions through halogen bonding at the electrophilic halogen moiety, hydrogen bonding at the acidic backbone protons, or anion-π interactions at the cationic imidazolium ring (pictured).
X-ray crystallography, NMR spectroscopy, and calorimetry, as well as molecular dynamics (MD) simulations were used to investigate the binding modes. The results allowed the team to exclude anion-π interactions. In addition, they showed that anion binding occurs either by pure halogen bonding or by halogen bonding and hydrogen bonding of similar strength. According to the researchers, this information could be useful for the development of anion receptors, anion transporters, and organocatalysts.
- The Interaction Modes of Haloimidazolium Salts in Solution,
Nils Schulz, Pandian Sokkar, Elric Engelage, Severin Schindler, Mate Erdelyi, Elsa Sanchez-Garcia, Stefan M. Huber,
Chem. Eur. J. 2017.
https://doi.org/10.1002/chem.201705032 - Cluster of Excellence RESOLV, Germany