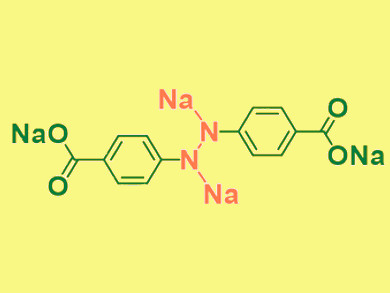
Organic materials are promising candidates for use in rechargeable batteries because they are lightweight, abundant, and low-cost. However, organic anode development is hampered by the low conductivity and high solubility of these materials—qualities that impact the lifetime and charging capabilities of a battery.
Chunsheng Wang, University of Maryland, College Park, MD, USA, and colleagues have synthesized organic azo compounds for use in high-performance sodium-ion batteries (SIBs). The electrochemical reduction/oxidation of azo compounds leads to reversible sodiation–desodiation of the azo group (sodiated form pictured). The aromatic structure of the azo compounds promotes conductivity, and two lipophobic carboxylate groups reduce the solubility in organic electrolytes.
An optimized compound, azobenzene 4,4’-dicarboxylic acid sodium salt, offers high conductivity, low solubility, and impressive electrochemical performance. The salt delivers reversible capacities of 170 mAh g–1 at 0.2 C, and a steady 98 mAh g–1 at 20 C for 2,000 cycles. Azo-compound-based batteries retain a high capacity for thousands of cycles, thereby satisfying the high energy and long lifetime requirements of renewable energy for smart grids.
- Reversible Redox Chemistry of Azo Compounds for Sodium-Ion Batteries,
Chao Luo, Gui-Liang Xu, Xiao Ji, Singyuk Hou, Long Chen, Fei Wang, Jianjun Jiang, Zonghai Chen, Yang Ren, Khalil Amine, Chunsheng Wang,
Angew. Chem. Int. Ed. 2018, 57, 2879–2883.
https://doi.org/10.1002/anie.201713417