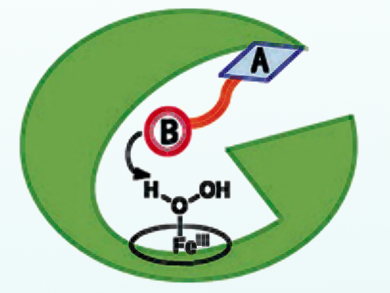
Cytochrome P450s are synthetically useful enzymes that can act as biocatalysts for the monooxygenation of various organic substrates such as hydroxylation, epoxidation, and sulfoxidation. However, their practical application is limited by their complicated electron-transport chains and the need for stoichiometric NAD(P)H (reduced nicotinamide adenine dinucleotide (phosphate)). An alternative is the use of H2O2, which would dramatically simplify the use of P450s, but is itself hampered by the poor activity of cytochromes in the presence of this oxidant.
Zhiqi Cong, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Shandong, and colleagues have rationally devised dual-functional small molecules (DFSMs) to facilitate the H2O2 activation process for P450BM3, one of the best-studied P450s. The DFSMs, such as N‐(ω‐imidazolyl fatty acyl)‐l‐amino acids, have an acyl amino acid group responsible for bounding to enzymeas an anchoring group (pictured as A), while an imidazolyl group (pictured as B) acts as a general acid–base catalyst in the activation of H2O2. This approach has resulted in greatly improved H2O2-driven monooxygenase activity towards unnatural substrates.
The scientists point out that their system is simple and practical in carrying out P450BM3-catalyzed oxidations using H2O2. The method is also promising for application to other P450s and for exploiting enzyme activity and function based on direct chemical intervention in the catalytic process.
- Dual-Functional Small Molecules for Generating an Efficient Cytochrome P450BM3 Peroxygenase,
Nana Ma, Zhifeng Chen, Jie Chen, Jingfei Chen, Cong Wang, Haifeng Zhou, Lishan Yao, Osami Shoji, Yoshihito Watanabe, Zhiqi Cong,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201801592