Resilient Materials
Porous compounds known as covalent organic frameworks (COFs) have shown stability against acid and alkali attack up to 18-molar sulfuric acid, 12-molar hydrochloric acid, and 9-molar sodium hydroxide solution. Such resilience bodes well for their use in a wide variety of applications, including in separation science and for the recovery of acids.
Porous compounds are nothing new: There are many natural examples of crystalline materials, such as zeolites, that contain pores and channels and that have been used as molecular sieves, as catalysts, as gas storage materials, and as the basis of chemical sensors. There are also many semi-synthetic versions of such materials, modifications of the natural structures as well as porous solids that have been designed and synthesized in the laboratory. Among the latter are metal-organic frameworks (MOFs) and covalent organic frameworks (COFs).
Overcoming Porous Problems
The problem with many porous crystalline solids is that they are rather flimsy. Some select structures hold up well to diverse challenges, but heat, acidity, and even just high levels of humidity can lead to the collapse of many porous solids. Rahul Banerjee, CSIR-National Chemical Laboratory, Pune, India, and Indian Institute of Science Education and Research (IISER), Kolkata, and colleagues hope to change that. They have developed what they refer to as a “rapid and scalable synthesis” of six new imine-linked highly porous and crystalline COFs.
The team describes COFs as “pristine sets of covalently linked, periodically ordered porous networks synthesized through simultaneous polymerization and crystallization of monomeric building blocks.” The reversibility of the reactions used to piece together the requisite building blocks from light elements (hydrogen, boron, carbon, oxygen, and nitrogen), such as boronic acid trimerization, boronate ester formation, or Schiff base formation, would suggest that COFs might be even more flimsy than their heavier MOF counterparts, which have metallic centers. Building a COF that can resist harsh conditions of strong acids, alkalis, and extreme humidity remained a significant challenge.
By using the enol-to-keto tautomerization phenomenon during the framework crystallization, the team found a way to improve COF stability. However, this process limited the stable COFs to β-keto-enamine structures, which limits the scope of building blocks and, thus, the range of possible applications. Imine-linked COFs, in contrast, would be far more versatile. Other researchers had explored imine-linked COFs, but Banerjee and colleagues believe they have side-stepped the various problems with earlier approaches such as the need for toxic solvents, synthetic complexity, and the lack of a way to quickly and easily scale up the reactions. Moreover, the new approach gives researchers the chance to fabricate the as-synthesized COFs into pellets, beads, or even membranes for various applications, while maintaining all the desirable properties.
Imine-Based COFs
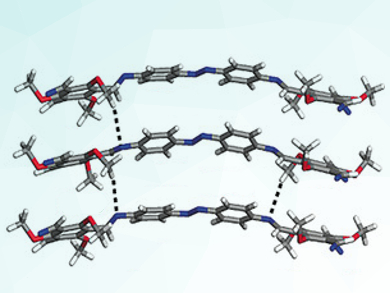
Key to the team’s success in making their stable COFs is an aldehyde, specifically 2,4,6-trimethoxy-1,3,5-benzenetricarbaldehyde (TpOMe). This aldehyde allowed them to construct six stable, imine-linked COFs (pictured) in a scalable manner, quickly and relatively easily using an approach known as p-toluenesulfonic acid-mediated solid-state mixing.
Team member Matthew Addicoat points out that this approach has several important advantages. “No solvent (solid state mixing) equates to no waste, which is much better for the environment,” he told ChemViews Magazine. He adds that “there are two benefits to using this particular aldehyde building block: the compound that forms the imine bonds that make the actual COF also forms a lot of hydrogen bonds with the neighboring COF layers.” Although hydrogen bonds are weaker than common covalent bonds, there are plenty of them in this COF and they essentially form a cage around the imine bond, so that the acid and water cannot get in to attack and break the bond. “Having proven that you can use hydrogen bonds to stabilize a COF, it can be generalized to any synthesis method with boronic acid, boronate, etc.,” Addicoat stated.
Ultra‐High Chemical Stability
The COFs are stable to all of the aforementioned acid and alkaline conditions as well as to boiling water and various common organic solvents over a period of several days. Tests show that the COFs can be used to recover 6-molar sulfuric acid from mixtures and can be fabricated into membranes to remove toxic substances from contaminated water. The material’s scalability and stability bode well for many other applications.
Addicoat adds that, “I think the big next step for porous materials, in general, is tailoring the framework to specific tasks, such as filtering toxic metals from water.” A specific COF might be constructed to remove mercury, arsenic, or other elements from contaminated water.
“This paper reports a very important advance in the construction of stable molecular frameworks,” explains Thomas Bein, University of Munich (LMU), Germany. “While numerous intriguing and beautiful porous molecular frameworks have been successfully created and published, it is their stability under relevant conditions that decides upon their impact regarding functionality and applications.” He adds that, “So far, only a small select group of frameworks meets such requirements, hence, any conceptual progress along this line will likely have an important impact.”
- Ultrastable Imine-Based Covalent Organic Frameworks for Sulfuric Acid Recovery: An Effect of Interlayer Hydrogen Bonding,
Arjun Halder, Suvendu Karak, Matthew Addicoat, Saibal Bera, Amit Chakraborty, Shebeeb H. Kunjattu, Pradip Pachfule, Thomas Heine, Rahul Banerjee,
Angew. Chem. Int. Ed. 2018, 57, 5797–5802.
https://doi.org/10.1002/anie.201802220