The site-specific chemical modification of proteins is a powerful method to understand and engineer protein function. One target for such modifications is the 5-hydroxytryptophan residue. This unnatural amino acid can be site-specifically incorporated into proteins expressed in both Escherichia coli and eukaryotic cells by genetic modifications.
Abhishek Chatterjee and colleagues, Boston College, MA, USA, have developed a chemoselective protein-labeling strategy targeted to the 5-hydroxytryptophan residue. The team combined the full-length protein, ferricyanide as a mild oxidant, and different aromatic amines as a labeling agent in an aqueous phosphate buffer (pH 7) on ice.
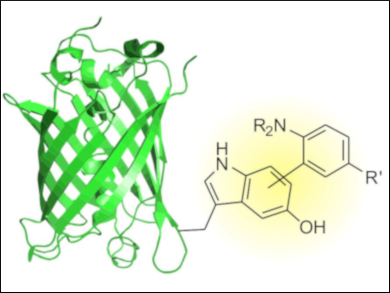
The electron‐rich 5‐hydroxyindole residue of 5‐hydroxytryptophan can be chemoselectively labeled with a variety of aromatic amines (example pictured) under mild conditions in an oxidative coupling reaction. The developed conjugation strategy might also be compatible with other established reactions commonly used for protein labeling.
- An Oxidative Bioconjugation Strategy Targeted to a Genetically Encoded 5-hydroxytryptophan,
Partha Sarathi Addy, James S. Italia, Abhishek Chatterjee,
ChemBioChem 2018.
https://doi.org/10.1002/cbic.201800111