Proton coupled electron transfer (PCET) is a critical process in many chemical reactions, e.g., in key steps in the function of Photosystem II in photosynthesis and in hydrocarbon combustion in a car’s engine. It is characterized by the transfer of a “net hydrogen atom”, a proton, and an electron. This can happen via many different routes, which cannot necessarily be easily distinguished from one another. This limits our theoretical understanding of many chemical reactions.
Theoretical Distinction
Johannes Klein, Rijksuniversiteit Groningen, The Netherlands, and Gerald Knizia, Pennsylvania State University, University Park, USA, have argued that it is not only feasible but could be fruitful to “directly analyze the electronic N-electron wave functions of these processes along their intrinsic reaction coordinate (IRC)” in order to establish the details of such transfer reactions.
Depending on the circumstances, processes covered by the umbrella term PCET range from the coupled but distinct transfer of a proton and an electron to the literal transfer of a hydrogen atom. The researchers propose that it is possible to establish which view of the process is most appropriate using direct computational analysis. For this, they use Intrinsic Bond Orbitals (IBOs), a simple but formally exact representation of the N-electron wave function which allows the visualization and interpretation of electron flow during a chemical reaction.
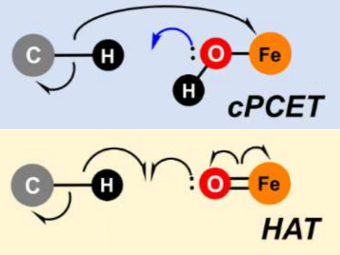
To demonstrate their idea, the team has turned to two model systems on opposite ends of the spectrum: first, a well-known and well-studied lipoxygenase with an Fe(III)-OH active site, which breaks one of the C–H bonds of arachidonic acid, and second, the high-valent oxoiron(IV) intermediate taurine dioxygenase (TauD-J), which oxidizes a C–H bond in taurine. By using the wave function’s intrinsic bond orbital (IBO) representation for these two systems, the researchers can then directly and unambiguously distinguish the common boundary cases (pictured): hydrogen atom transfer (HAT) and concerted PCET (cPCET).
This is important because identifying which of two apparent mechanistic scenarios is actually taking place is usually a serious challenge because one is attempting to distinguish between the same net transfer of one electron and one proton in either case. The mechanism cannot be derived from simple knowledge of the properties of the reactants and products.
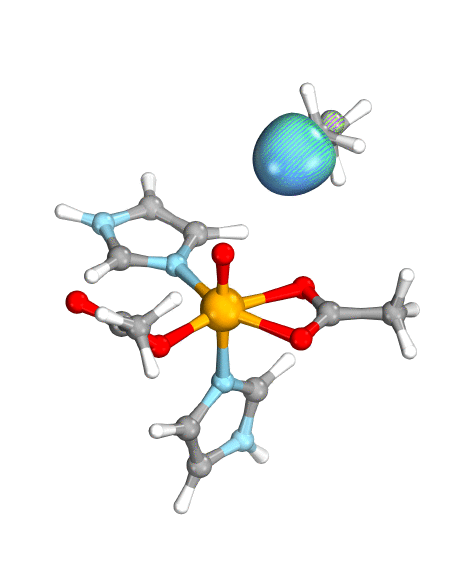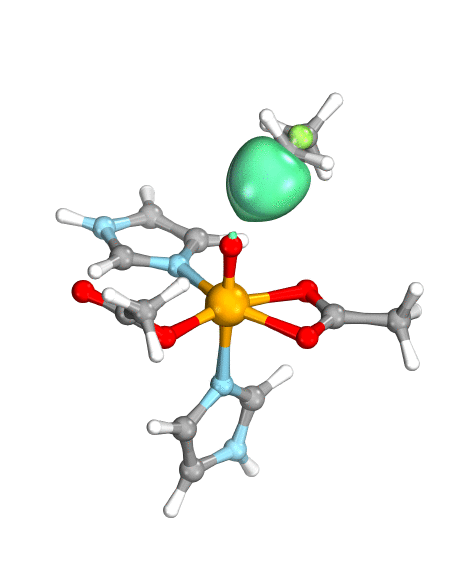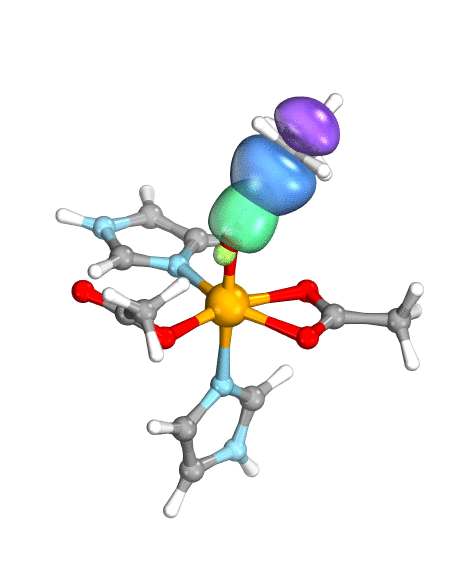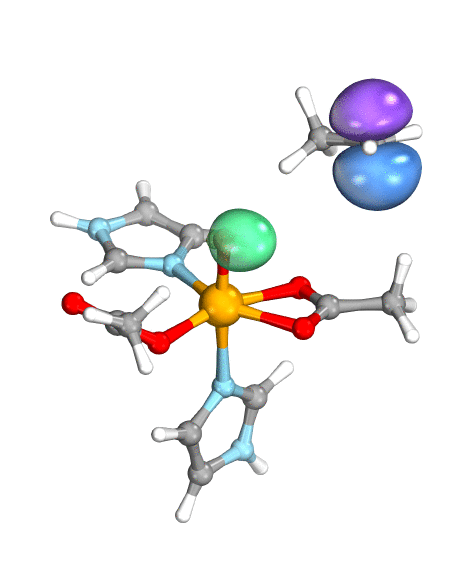
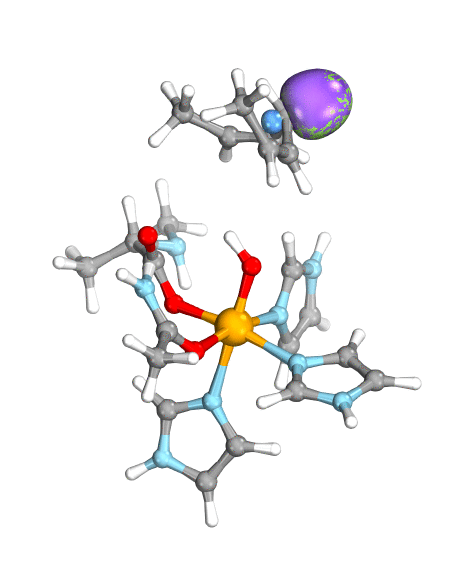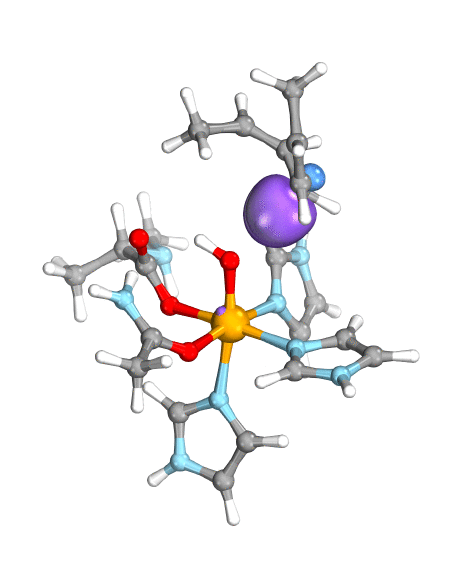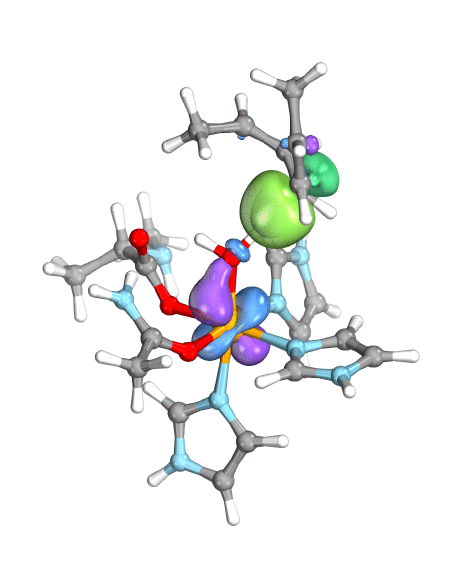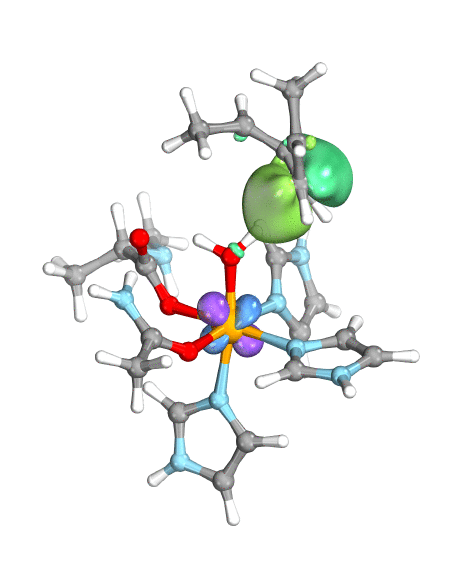
Figure. Left: Hydrogen atom transfer (HAT) by the high-valent intermediate J of a taurine dioxygenase (TauD-J) model. The substrate C–H bond breaks homolytically. One of its electrons (green) travels together with the proton as TauD-J is reduced, forming the new O–H bond—we have a hydrogen atom transfer.
Right: cPCET in a lipoxygenase model complex. Again a C–H bond homolytically breaks. But the electron (blue) is directly transferred to the iron of the enzyme’s active site, while an oxygen ion pair morphs into the new O–H bond. So we have a proton and electron transfer in concert, but not together—a cPCET event. © J. E. M. N. Klein, University of Groningen
IBO Success
“We have found a way of establishing which of the distinct cases is actually happening in a concrete reaction,” Knizia told ChemViews Magazine. “This is a frequent matter of intense debate, because the processes are currently mostly interpreted based on very indirect experimental or theoretical inference, which can sometimes be contradictory or misleading. Our way directly computes this information.”
Based on their success with these two prototypical model systems for splitting C–H bonds, the team concludes that IBOs can be used to readily examine the electron flow of open-shell reactions. They suggest this makes their approach a straightforward tool for differentiating electronic mechanisms—even in such complex reactions. “We, therefore, believe this approach may shed light into many other challenging transformations,” the team adds.
- cPCET vs. HAT: A Direct Theoretical Method for Distinguishing X-H Bond Activation Mechanisms,
Johannes E. M. N. Klein, Gerald Knizia,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201805511
Also of Interest
- Electron Flow in Reaction Mechanisms-Revealed from First Principles,
Gerald Knizia, Johannes E. M. N. Klein,
Angew. Chem. Int. Ed. 2015, 54, 5518–5522.
https://doi.org/10.1002/anie.201410637
A Perspective on PCET Including Alternative Theoretical Approaches
- Proton-Coupled Electron Transfer: Moving Together and Charging Forward,
Sharon Hammes-Schiffer,
J. Am. Chem. Soc. 2015, 137, 8860–8871.
https://doi.org/10.1021/jacs.5b04087