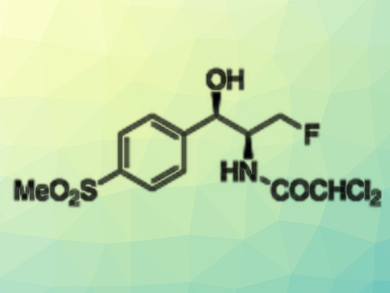
Fluorinated Antibiotics
Fluorinated structures make up more than 20 % of modern drugs, but benign and fast fluorination schemes are scarce. Fuli Zhang, Shaoxin Chen, China State Institute of Pharmaceutical Industry, Shanghai, and colleagues have combined dynamic kinetic resolution and nucleophilic fluorination for the asymmetric synthesis of florfenicol, a veterinary antibiotic. After enzymatic resolution of the racemate, two different fluorination strategies both proved effective and fast. One is especially interesting for industrial production.
Fluorine substituents have many useful effects in drugs. Often, fluorinated compounds are more lipophilic, penetrate more easily in the cells, and enzymes may find it harder to degrade them. For example, the fluorinated antibiotic florfenicol is more active than thiamphenicol, to which it is related by a terminal fluorine instead of a hydroxy group. Florfenicol and thiamphenicol, which are both variants of chloramphenicol, are used against conjunctivitis and respiratory diseases in cattle.
Greener Processes
Despite the relevance of fluorination, many industrial fluorination strategies still involve aggressive and harmful reagents. In the case of florfenicol, not only is the fluorinating agent expensive and corrosive, but the preparation of the two stereocenters also produces a high amount of waste. These issues prompted the researchers to look for more environmentally benign processes. To synthesize florfenicol, they had to construct two adjacent stereocenters of the cis-1,2-amino alcohol and introduce a terminal fluorine atom.
The scientists chose to install the fluorine in a last, separate step because the two asymmetric carbon atoms can be conveniently prepared by dynamic kinetic resolution. Kinetic resolution means that two enantiomers can be separated according to their different reactivity. The process is called “dynamic” when the two enantiomers can racemize, that is, interconvert. Then, the faster reacting enantiomer can be isolated with maximal yield.
Kinetic Resolution with Ketoreductases
The team designed a dynamic reductive kinetic resolution using ketoreductase enzymes as biocatalysts and glucose as the hydride source. Having established the two stereocenters by this bioenzymatic route, they explored possible fluorination reactions. Two routes of nucleophilic fluorination proved especially convincing, and both routes use amine hydrofluoride as the fluorinating reagent, which is mild and selective and can be handled in glass equipment.
For industrial manufacture, the researchers also have a “favorite”. The route involving a cyclic sulfate intermediate outcompeted the other one containing an intermediate aziridine, a three-membered nitrogen-containing ring. The chemo-enzymatic sulfate route produced florfenicol in only five steps and showed high yield and flexibility as well as acceptable environmental and health safety. The next step will be the further exploration of the reductase enzymes in other dynamic bioreduction syntheses, the team states.
- Asymmetric Synthesis of Florfenicol by Dynamic Reductive Kinetic Resolution with Ketoreductases,
Jie Zou, Guowei Ni, Jiawei Tang, Jun Yu, Luobin Jiang, Dianwen Ju, Fuli Zhang, Shaoxin Chen,
Eur. J. Org. Chem. 2018.
https://doi.org/10.1002/ejoc.201800658