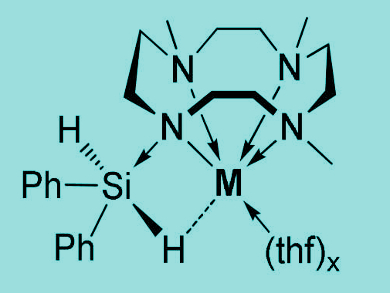
Hypervalent hydrosilicates are anionic silicon compounds with a reactive silicon–hydrogen bond. They are more reactive than tetravalent hydrosilanes and allow the reduction of unsaturated organic compounds or the hydrodefluorination of fluorobenzenes. However, isolated examples have been scarce due to the compounds’ instability. There had been only one example of a structurally characterized hydrosilicate so far.
Jun Okuda and colleagues, RWTH Aachen University, Germany, have synthesized crystalline hypervalent dihydridosilicates supported by light alkali metal amides (pictured, M = Li, Na, K). The team treated light alkali metal amides [M(Me3TACD)]n (M = Li, Na ,K; Me3TACDH = 1,4,7‐trimethyl‐1,4,7,10‐tetraazacyclododecane) with H2SiPh2 in tetrahydrofuran (THF) to give the desired products.
The compounds were fully characterized by elemental analysis, NMR and infrared (IR) spectroscopy, and single-crystal X-ray crystallography. Key to the stabilization is the use of the macrocyclic ligand tetraazacyclododecane (cyclen).
The lithium complex catalyzes the hydrosilylation of styrene derivatives. The sodium and potassium derivatives display a direct interaction between one of the hydrides on silicon and the metal. These compounds can act as hydride transfer reagents.
- Hypervalent Hydrosilicates Connected to Light Alkali Metal Amides: Synthesis, Structure, and Hydrosilylation Catalysis,
Danny Schuhknecht, Valeri Leich, Thomas P. Spaniol, Jun Okuda,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201803373