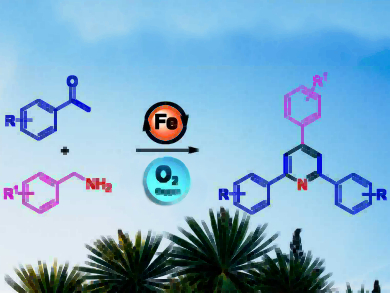
2,4,6-Triarylpyridines and their derivatives (also called “Kröhnke pyridines”, pictured right) have applications in medicinal chemistry and advanced materials. However, syntheses for this type of compound that are simple, cost-effective, and environmentally benign are still rare.
Kovuru Gopalaiah, University of Delhi, India, and colleagues have developed an efficient iron-catalyzed aerobic oxidative tandem reaction for the synthesis of 2,4,6-trisubstituted pyridines. The approach uses commercially available starting materials such as benzylamines and arylalkylketones, a cheap FeBr2 catalyst, molecular oxygen as a green oxidant, and no solvent.
The reaction has a broad substrate scope and no hazardous byproducts. The proposed mechanism involves an oxidative self-condensation of benzylamine that releases ammonia, a coupling of the resulting imine to the enol form of the ketone reactant, a condensation with ammonia to form the pyridine ring, and a re-aromatization to obtain the desired product.
- Iron-Catalyzed Aerobic Oxidative Cleavage and Construction of C–N Bonds: A Facile Method for Synthesis of 2,4,6-Trisubstituted Pyridines,
Kovuru Gopalaiah, Devarapalli Chenna Rao, Kuldeep Mahiya, Ankit Tiwari,
Asian J. Org. Chem. 2018.
https://doi.org/10.1002/ajoc.201800312