Hydrogen can be used as an environmentally friendly fuel. Electrochemical water splitting is one of the most promising approaches to generate hydrogen. However, in order to increase the efficiency of this process, the overpotential of electrochemical processes at both cathode and anode need to be reduced.
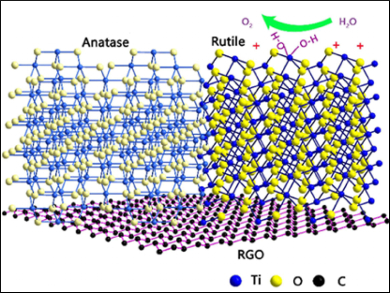
Kaibin Tang, University of Science and Technology of China, Hefei, and colleagues have developed nanostructures, composed of both rutile and anatase titania (TiO2) and reduced graphene oxide (RGO), as catalysts for the oxygen evolution reaction. The team first synthesized an anatase/RGO nanocomposite via a one-step solvothermal method using TiS2 and graphene oxide solutions. The resulting nanocomposites were then annealed to induce a partial phase transition from anatase to rutile.
The charge transfer between rutile and anatase, as well as between TiO2 and RGO, enhances the adsorption of hydroxyl species, and thus, the electrochemical activity. The nanocomposites exhibit a current density of 10 mA cm–2 at a low overpotential of 283 mV. The performance is superior to previously used TiO2, which has an overpotential of 600 mV.
- Component-Tunable Rutile-Anatase TiO2/Reduced Graphene Oxide Nanocomposites for Enhancement of Electrocatalytic Oxygen Evolution,
Yiwei Hu, Tao Ding, Kailong Zhang, Biao Li, Baichuan Zhu, Kaibin Tang,
ChemNanoMat 2018.
https://doi.org/10.1002/cnma.201800252