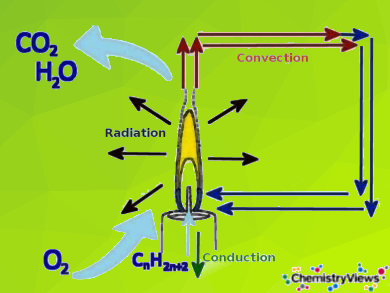
Candle flames are considered laminar diffusion flames. By diffusion flame, it is meant that the fuel (here the candle wax in the form of various hydrocarbons) and the oxygen (from the air) required for the combustion are brought separately to the reaction zone (here the wick).
The mixture of fuel and oxygen occures by convection and diffusion: The liquid fuel rises by capillary forces in the wick. Here it evaporates due to the high flame temperature. The hot gas rises and mixes with the ambient air through diffusion. In addition, colder air flows into the lower part of the flame through convection. Here it also mixes with the gaseous fuel. The actual combustion reaction preferably starts in a zone of the flame above the wick.
How long does it take for a candle to burn down?
- Smaller candles with smaller wicks will burn at a rate of 7 to 9 hours per 28 g of wax used.
- Larger candles with larger wicks consume wax at a faster rate. The larger wicks can be expected to yield 5 to 7 hours per 28 g of wax used.
What does the burning time depend on?
- Size of the candle
- Shape of the candle
With thick candles occasionally “wall parts” remain because they are not heated enough by the flame. - Movement of air in the environment
- Amount of oxygen in the environment
- Temperature of the environment
- Hardness of the wax.
- Type of wax used
Candle wax consists of a mixture of saturated and unsaturated long-chain hydrocarbons, acids, alcohols, and esters. The mixture of these components determines the wax type. Depending on the mixture, the dominant reactions and thus their combustion rates differ. - The additives used on the wax
- The size of the wick
- The material of the wick
- The amount of fragrance in the candle
With so many variables, a burn test is often required to get an accurate estimate of the burn time of a candle. A first hint is given by the weight of the candle. Or you can just wait and see while enjoying the beautiful light of the candle.
Clever Picture: What Makes a Candle Flame?
- 06 December 2011
- The different reaction zones of a candle flame and its heat and mass transfer pathways
Video: Where is the Heat of a Candle Flame?
- 08 December 2016
- With an experiment of Michael Faraday, we examine where the heat of a candle is
Video: Chalk as a Wick
- 7 December 2019
As with a wick, liquid fuel is supplied to the flame by capillary forces against the force of gravity
Focus Article: Chemistry of the Christmas Candle
- 02 November 2011
- When we light a candle, the chemistry we are pursuing is not only especially beautiful, but also especially complex
- Die Qual der Wahl an Weihnachten,
Michael Vollmer, Klaus‐Peter Möllmann,
Phys. Unserer Zeit 2018.
https://doi.org/10.1002/piuz.201801528







This website is so helpful. I gained so much form it and I hope other people will do as well.