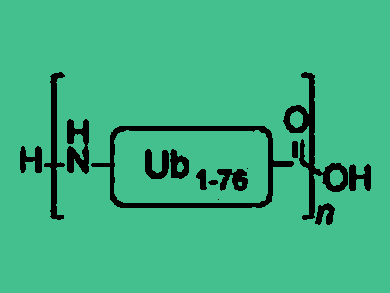
The enzyme-mediated construction of pol-ubiquitin (Ub) chains on target proteins leads to a variety of cellular responses and is involved in processes ranging from protein degradation to cell cycle control. This complex post-translational modification system is under intense investigation, and the chemical generation of specific Ub chains may help to further understand its important biological function.
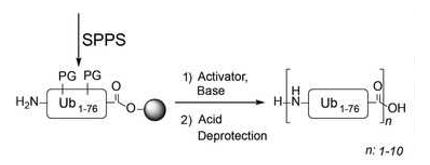
Gerbrand J. van der Heden van Noort and colleagues, Leiden University Medical Centre, The Netherlands, have discovered that native methionine-1-linked polymeric ubiquitin chains can be constructed in a single chemical reaction. Solid-phase peptide synthesis (SPPS) of Ub can be used to chemically prepare the Ub protein. Mutants of this protein can be used to make Ub-based tools or diUb species in a native chemical ligation approach. Constructing larger poly Ub chains, however, remains a challenge.
The researchers showed that performing a polymerization reaction using resin-bound Ub straight after SPPS results in natural poly Ub chains linked via Methionine-1 with a chain length of up to 10 Ub residues (pictured below). This approach towards large poly Ub chains may lead to the development of new tools to study this important post-translational modification.
- One-Step Chemical Synthesis of Native Met1-Linked Poly-Ubiquitin Chains,
Gerbrand J. van der Heden van Noort, Cami Talavera Ormeño, Duco van Dalen, Huib Ovaa,
ChemBioChem 2018.
https://doi.org/10.1002/cbic.201800520