Peroxisome proliferator‐activated receptor gamma (PPARγ) is a protein which controls the expression of genes involved in, e.g, metabolic processes. PPARγ is a therapeutic target for the treatment of type 2 diabetes. However, PPARγ-targeting drugs, such as rosiglitazone and pioglitazone, are less often used because of adverse effects caused by their mechanism of action.
Seung Bum Park, Seoul National University, Korea, and colleagues have developed a PPARγ antagonistic ligand which inhibits the phosphorylation of PPARγ at the residue Ser273. Blocking Ser273 phosphorylation has been proposed as the key for developing insulin‐sensitizing effects in PPARγ‐targeting drugs.
The team designed and synthesized a series of PPARγ phosphorylation inhibitor analogues, based on the analysis of co-crystal structures of PPARγ and its known ligands. Starting from these known compounds, they performed systematic structural modifications, stability tests, and computational docking studies.
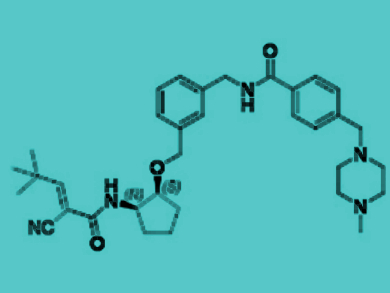
Using this process, the reserchers developed SB1495 (pictured). It contains a tert-butyl substituted aliphatic cyanoacrylamide moiety and acts as a reversible covalent inhibitor of PPARγ phosphorylation without the mechanism that triggers the adverse effcts of known PPARγ-targeting drugs. The biological activity of SB1495 was demonstrated in cells as well as in a mouse model and the results show its potential for further biological applications.
- Rational design and synthesis of reversible covalent PPARγ antagonistic ligands inhibiting Ser273 phosphorylation,
Hyunsoo Kim, Ala Jo, Sun-sil Choi, Hyunsung Nam, Wan Gi Byun, Hwan Bae, Jang Hyun Choi, Seung Bum Park,
Asian J. Org. Chem. 2018.
https://doi.org/10.1002/ajoc.201800668