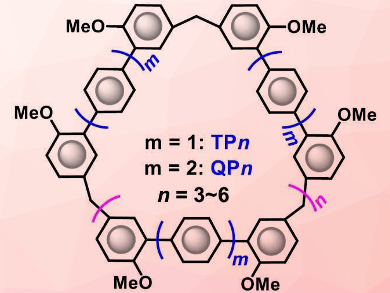
Macrocycles are important compounds in supramolecular chemistry. The cavities of classic macrocyclic receptors are, generally smaller than 10 Å. It is challenging to synthesize macrocycles with larger cavities.
Chunju Li, Shanghai University, China, and colleagues have developed two new classes of large macrocycles, terphen[n]arenes (TPn) and quaterphen[n]arenes (QPn) (pictured, with n = 3–6) which consist mainly of benzene rings that are either directly connected or linked via methylene bridges. These structures can be accessed through one-step condensation reactions of 2,2”‐dimethoxy terphenyl or 2,2”’‐dimethoxy quaterphenyl monomers in good yields.
The products have much larger cavities than traditional synthetic receptors and show interesting self-assembly properties. The cavity size of QP6 is larger than 3.0 nm. The cyclic pentamers and hexamers—TP5, TP6, QP5, and QP6—can form supramolecular gels. This process is driven by π⋅⋅⋅π stacking interactions. The dried gel materials can effectively capture iodine in water or from the vapor phase and, thus, could have applications in pollutant sequestration.
- Terphen[n]arenes and Quaterphen[n]arenes (n=3-6): One-Pot Synthesis, Self-Assembly into Supramolecular Gels, and Iodine Capture,
Bin Li, Bin Wang, Xiayang Huang, Lu Dai, Lei Cui, Jian Li, Xueshun Jia, Chunju Li,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201813972