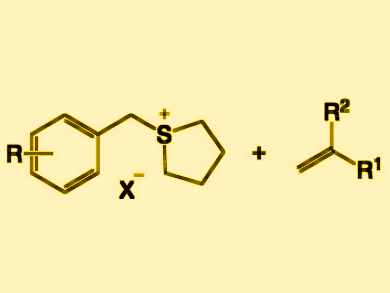
The Mizoroki–Heck reaction is an alkenylation reaction of organic halides using alkenes. The alkenylation of organosulfur compounds instead of organic halides is challenging due to the catalyst-poisoning nature of the anionic sulfur species generated in the course of the reaction.
Hideki Yorimitsu, Kyoto University, Japan, and colleagues have developed a photoredox-catalyzed alkenylation of benzylsulfonium salts via cleavage of their C–S bonds. The team used fac-Ir(ppy)3 (ppy = 2-phenylpyridine) as a catalyst, Na2CO3 as a base, and N-methylpyrrolidone as a solvent to react several benzylsulfonium salts (pictured left) and styrenes (pictured right) under blue light-emitting diode (LED) irradiation. The benzylsulfonium reactants can be readily prepared from the corresponding benzyl alcohols.
The desired allylbenzene products were obtained in moderate to good yields. The leaving groups are neutral sulfides, which do not poison the catalyst as easily as anionic species. The reaction tolerates a variety of functional groups, such as halogen, ester, and cyano groups.
- Photoredox-Catalyzed Alkenylation of Benzylsulfonium Salts,
Shinya Otsuka, Keisuke Nogi, Tomislav Rovis, Hideki Yorimitsu,
Chem. Asian J. 2019.
https://doi.org/10.1002/asia.201801732