Transition-metal-catalyzed asymmetric allylic substitution reactions are useful for the construction of chiral motifs that are commonly found in natural products and pharmaceutically active compounds. The use of benzylic nucleophiles in this type of reaction, such as those derived from simple methyl azaarenes, is challenging but could be useful.
Shu-Li You, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and colleagues have developed an iridium-catalyzed asymmetric allylic alkylation reaction of methyl azaarenes (pictured). In the reaction, the Knochel reagent TMPZnBr·LiBr is a crucial base for generating benzylic nucleophiles in situ. The reaction was carried out in the presence of a catalyst comprised of [Ir(cod)Cl]2 (cod = 1,5-cyclooctadiene) and a chiral Carreira (phosphoramidite, olefin) ligand at ambient temperature.
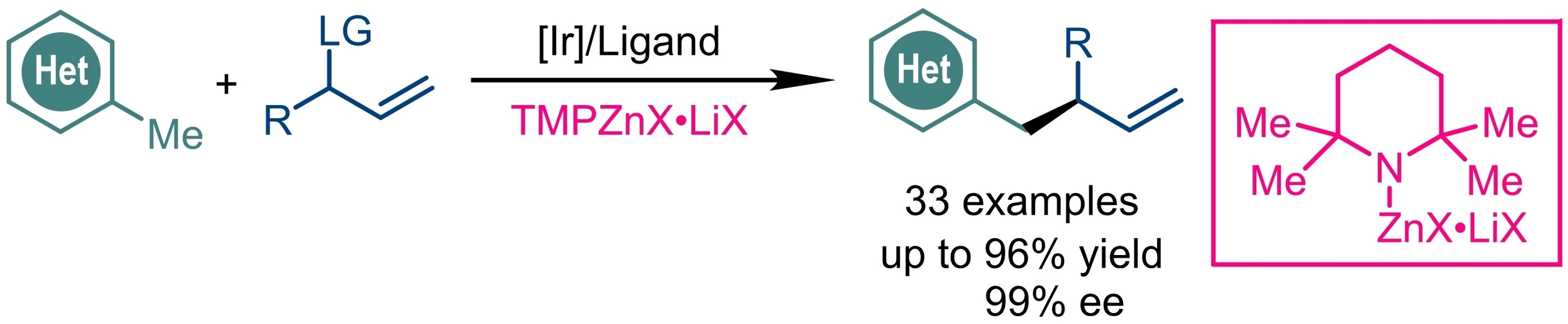
A variety of methyl azaarenes, including (benzo)thiazole, oxazole, benzoimidazole, pyridine, and (iso)quinoline are compatible with the reaction. The desired products were obtained in high yields (up to 96 %) and high enantioselectivities (up to 99 %). The reaction was scaled up to the 2 mmol scale, and the team demonstrated the synthetic potential of the method by a concise synthesis of a chiral allosteric protein kinase modulator in 63 % yield and 82 % ee.
- Iridium‐Catalyzed Asymmetric Allylic Substitution of Methyl Azaarenes,
Xi‐Jia Liu, Wen‐Yun Zhang, Chao Zheng, Shu‐Li You,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202200164