Fluorinated compounds are useful for a wide range of applications, from materials to life sciences. The properties of molecules can often be tuned for specific uses by incorporating a fluorinated group. They can be further changed by the association of fluoroalkyl groups to a chalcogen (O, S, and Se). However, the preparation of such fluoroalkylseleno compounds is still challenging in some cases.
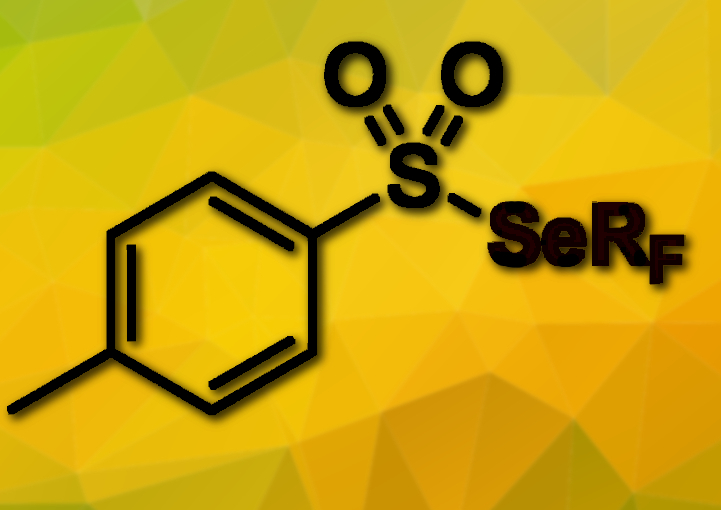
Thierry Billard, Fabien Toulgoat, and colleagues, CNRS and CPE Lyon, France have developed a new route to introduce fluoroalkylseleno groups (RFSe) to various alkyl halides. The reaction is based on the reduction of fluoroalkylselenotoluenesulfonates (RFSeTs, pictured) by an organic electron donor, tetrakis(dimethylamino)ethylene (TDAE). This reaction generates anionic species that then react with alkyl halides in nucleophilic substitutions.
Trifluoromethylseleno and perfluoroalkylseleno compounds were obtained in moderate to good yields. This strategy shows the versatility of fluoroalkylselenotoluenesulfonates (RFSeTs) reagents: They had already been used for electrophilic or radical processes and can now be also used for nucleophilic reactions.
- Umpolung Reactivity of Fluoroalkylselenotoluenesulfonates: Towards a Versatile Reagent,
Clément Ghiazza, Alex Kataria, Anis Tlili, Fabien Toulgoat, Thierry Billard,
Asian J. Org. Chem. 2019.
https://doi.org/10.1002/ajoc.201900027The article is part of a special issue on organofluorine chemistry.