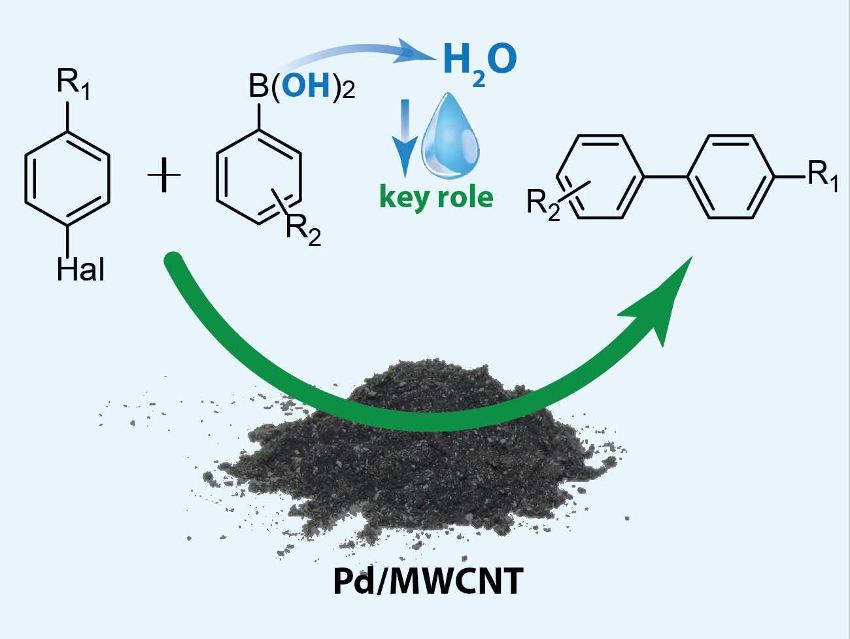
The Suzuki-Miyaura reaction is useful, e.g., for the production of a variety of pharmaceuticals. However, large amounts of solvents are needed to obtain even a small amount of pure product—both in the reaction and during workup. In addition to the problems these solvents pose for sustainability, cost-control, and the environment, they also cause leaching of supported palladium catalysts in these reactions. All of these issues could be resolved by converting the process to the solid state.
Evgeniy Pentsak and Valentine P. Ananikov, Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Moscow, have shown that one possible solid substrate for the Suzuki-Miyaura reaction, phenylboronic acid, can effectively serve as a source of water molecules (pictured above). These water molecules form as a by-product of a side reaction, the trimerization of phenylboronic acid. This small amount of water can play a key role in the desired transformation, making it a pseudo-solid-state reaction.
The team reacted solid aryl halides with phenylboronic acid in the absence of any liquids. The reaction was catalyzed by palladium nanoparticles supported on multi-walled carbon nanotubes (Pd NPs/MWCNT) with K2CO3 as a base. The reaction gave yields up to 97 %. Using control experiments, the researchers could show that the water from phenylboronic acid is crucial to the reaction.
The resulting pseudo-solid-state reaction still avoids the use of external solvents. In addition, the product can be easily separated by sublimation and the catalyst can be reused. The use of solvents is, thus, avoided at all stages, which is promising for a green approach to the Suzuki-Miyaura reaction.
- Pseudo-Solid-State Suzuki-Miyaura Reaction and the Role of Water Formed by Dehydration of Arylboronic Acids,
Evgeniy Pentsak, Valentine P. Ananikov,
Eur. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201900410