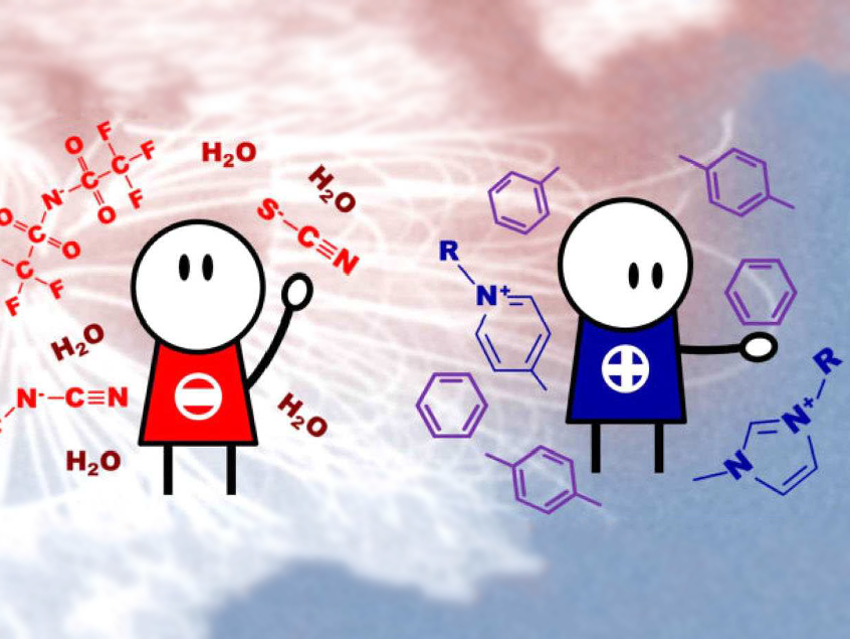
Ionic liquids (ILs) receive a great deal of interest as solvents with unique properties. The use of ILs in the separation of aromatic from aliphatic species has potential industrial applications.
Robin Rogers, University of Alabama, Tuscaloosa, USA, and colleagues have investigated the chemical interactions between aromatic molecules and ILs, and the ILs’ ability to preferentially solubilize aromatic compounds. The team has studied the strength of the chemical interactions between aromatic species/water and ten imidazolium- and pyridinium-based ILs with four different anions using NMR spectroscopy. The anions are thiocyanate [SCN]−, dicyanamide [DCA]−, tricyanomethanide [TCM]−, and bis(trifuoromethylsulfonyl)imide [NTf2]−.
They found that the structure of the anion has the main influences on the interaction strength, as it modifies the anion-cation strength of the interaction. However, anion-cation interactions are not directly correlated to the maximum aromatic solubility. The aromatic solubility is also affected by steric effects and the number of interactions that the aromatic molecules can establish with the ions. The aromatic solubility is also decreased by higher water content in the ILs. The water-anion interactions are stronger than water-cation interactions. A larger number of cyano groups in the anion structure leads to stronger aromatic/ion interactions and weaker water/ion interactions due to the higher acidity and hydrophobicity of the anions. This results in better aromatic separation.
According to the researchers, the study suggests that the best ILs for aromatic extraction and separation are those whose chemical structure include pyridinium cations and [TCM]– or [NTf2]– anions. The study provides useful information for the development of solvents for separation processes.
- Insights into Ionic Liquid/Aromatic Systems from NMR Spectroscopy: How Water Affects Solubility and Intermolecular Interactions,
Noemí Delgado‐Mellado, Julián García, Francisco Rodríguez, Robin D. Rogers,
ChemPlusChem 2019.
https://doi.org/10.1002/cplu.201900192


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
