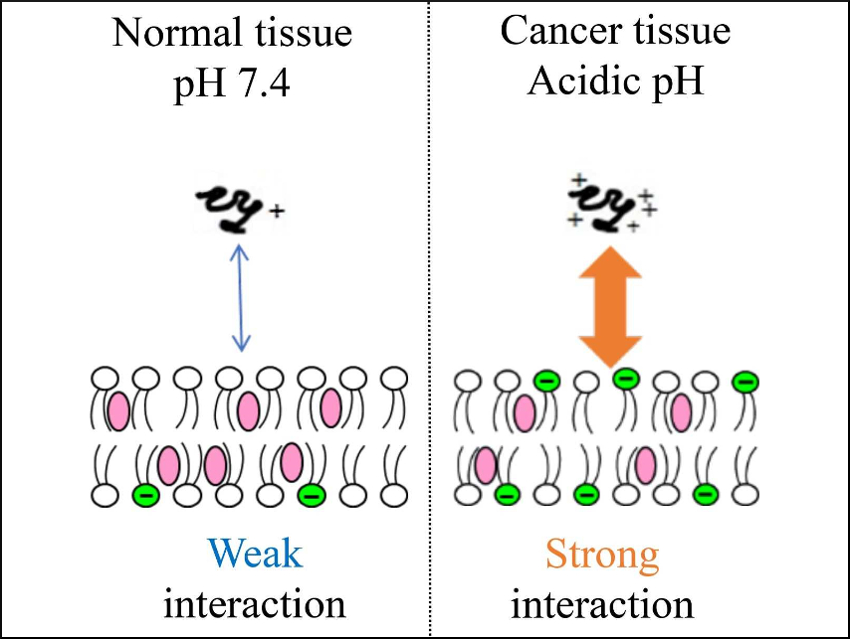
Side effects and drug resistances are two major obstacles for cancer chemotherapy. Cationic antimicrobial peptides are promising anti-cancer drug candidates. They preferentially bind to cancer cell membranes, which are more negatively charged than those of normal cells. The peptides then disrupt the membranes and kill the cells.
Katsumi Matsuzaki, Kyoto University, Japan, and colleagues have developed anti-cancer peptides which respond to acidic pH values. The peptides are based on the antimicrobial peptide magainin 2. They were modified by replacing lysine residues with the unnatural amino acid 2,3-diaminopropionic acid (Dap), which has a pKa value of ~6.3. The pH value in tumor tissue (5.6–6.9) is lower than that in normal tissue (~7.4). The modified peptides have a positive charge in such weakly acidic environments and, thus, are more active in tumor environments with negatively charged cell membranes.
The best peptide (with eight Dap residues) killed multidrug-resistant cancer cells in weakly acidic environments at more than ten times smaller concentrations than at pH 7.4. Furthermore, the peptide was almost nontoxic against normal cells at physiological pH. Incorporation of Dap residues into anticancer peptides could, thus, lead to a new mechanism of cancer tissue targeting.
- Endowment of pH responsivity to anticancer peptides by introducing 2,3-diaminopropionic acid residues,
Naoto Tanishiki, Yoshiaki Yano, Katsumi Matsuzaki,
ChemBioChem 2019.
https://doi.org/10.1002/cbic.201900226