Dr. Deanne Nolan, Deputy Editor of ChemPhotoChem, talked to Professor Alexander Heckel, University of Frankfurt am Main, Germany, about his work on a new universal chemical actinometer, a device used to calibrate light sources in photochemistry. The work was recently published in ChemPhotoChem.
Heckel developed a broadly absorbing indolyl-fulgide photoswitch. The photoswitch was used as an actinometer to determine photon fluxes over a broad range of wavelengths from the UV to the near-infrared (NIR) regions of the electromagnetic spectrum.
Would you please tell us what an actinometer is used for?
Photochemistry is a wonderful side-branch of (organic) chemistry which comes with its own rules and unique opportunities—such as the possibility to control molecular reactions in time and space. When it comes to designing and performing photochemical reactions, one can think of photons as a regular reagent which can be quantified in moles (also referred to as an “Einstein” here).
Unless used in excess, one needs to calibrate the light source in order to know how many photons end up in the sample under the given irradiation conditions. This process is called actinometry. It is as fundamental to photochemistry as the use of balances for weighing the correct amount of normal reagents.
What are your key findings?
We could show that a particular fulgide derivative [Editor’s note: pictured below] is an excellent substance for the quick and easy calibration of light sources. We measured the quantum yields for a large set of wavelengths and give detailed practical procedures on how to apply the method. We also suggest new ways for the mathematical treatment of the results.
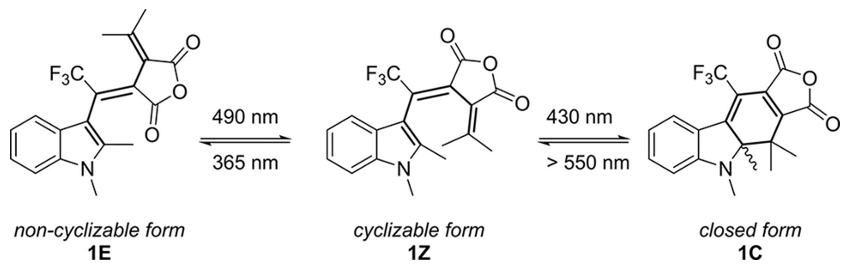
What distinguishes this chemical actinometry system from current methods for the determination of incident light intensity? Why did you choose a fulgide derivative for this purpose?
Many other actinometry methods have a narrow wavelength range, use compounds which are not readily available, or are experimentally cumbersome. The fulgide derivative we used is a perfect photoswitch: practically, it can be regarded as a two-state system which can be switched to either state almost completely (high photoswitching amplitude). The thermal conversion between these states is forbidden according to the Woodward–Hoffmann rules (high thermal stability).
Unlike with other photoswitches, this particular fulgide can be photoswitched many times without degradation (high fatigue resistance). Finally, its absorbance bands are well-separated and spread over a broad spectral range. All of these features make this fulgide derivative the most readily available well-behaved photoactive compound we know. Hence, we thought it would be ideal for calibrating light sources.
Can you tell us please about the motivation behind this study?
While the fulgide itself is very easy to use, it is a small part of a bigger scheme: For the last couple of years, we have been developing a comprehensive benchtop experimental photochemistry unit that is built from commercially available parts. Its goal is to address as many of the everyday needs of the photochemist on the (sub)second timescale as possible.
This system, which we call PHITS, can control several LED light sources, the reaction temperature, and the pH value. It has an adaptive mechanistic reaction scheme and allows a seamless transition from simulation to measurement, taking into account up to 16 reaction partners. To us, this level of complexity became necessary once we entered the field of developing spiropyran/merocyanine photoswitches. As part of this endeavor, using the presented fulgide derivative, the calibration of a light source is now only a matter of minutes and one does not even have to precisely weigh in the amount of fulgide needed.
As part of this work, you have also provided recommended procedures for undertaking actinometry using this photoswitch. Will it be possible to apply this technique across multiple fields of research?
While our motivation was a different one, the fulgide derivative is so robust that it can be used in every laboratory. Hence, we provide two procedures—a simple one and another one for the more ambitious user.
What are your next plans for this fulgide derivative?
This photoswitch is so well-behaved that it will become the workhorse of our practical course in optochemical biology.
The article they talked about
- A Robust, Broadly Absorbing Fulgide Derivative as a Universal Chemical Actinometer for the UV to NIR Region,
Matiss Reinfelds, Volker Hermanns, Thomas Halbritter, Josef Wachtveitl, Markus Braun, Tomáš Slanina, Alexander Heckel,
ChemPhotoChem 2019.
https://doi.org/10.1002/cptc.201900010The article is part of a ChemPhotoChem special issue on Photoresponsive Molecular Switches and Machines


