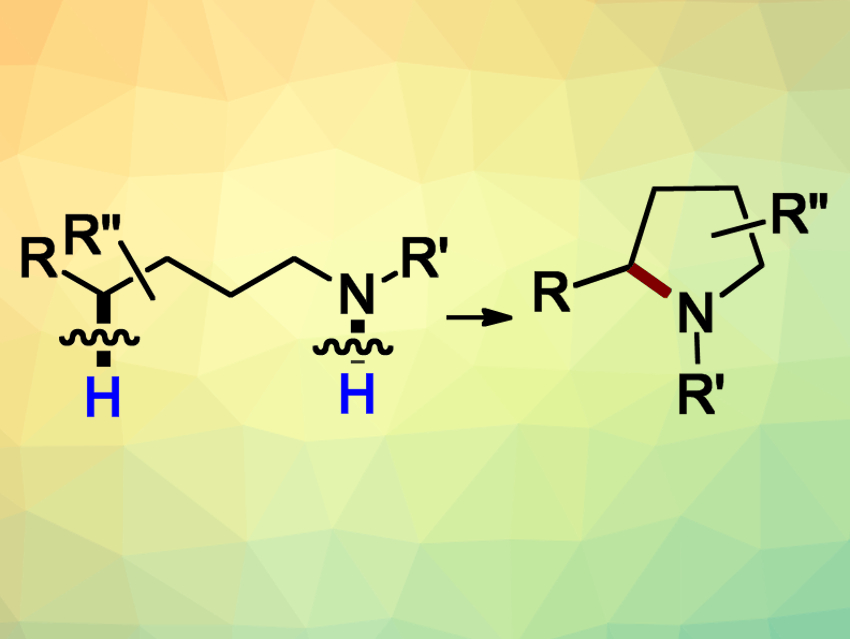
In order to access N-heterocyclic pyrrolidines (pictured) and piperidines, organic chemists often use the HLF (Hofmann–Löffler–Freytag) reaction (pictured). This reaction converts readily available primary aliphatic amines into their cyclic secondary counterparts. The cyclization is based on the generation of N-centered radicals, followed by a hydrogen atom transfer (HAT) and the ring closure. Despite the great potential of this reaction, there are still limitations regarding the generation of the initial radical species. Often, strong oxidants or harsh conditions have to be used.
Magnus Rueping, King Abdullah University of Science and Technology (KAUST), Thuwal, Saudi Arabia, and RWTH Aachen University, Germany, and colleagues have investigated the possibility of electrochemically generating N-centered radicals for the synthesis of nitrogen heterocycles. They found that, using a mild electrochemical procedure, the N-centered radicals can be directly formed from tosyl-protected amines. The reaction can be performed in undivided electrochemical cells with cheap and readily available graphite and stainless steel electrodes. The N-radical species are generated at the anode and the base required for the reaction is generated at the cathode.
The team prepared a broad range of valuable pyrrolidines in good yields and with high chemoselectivity. The approach is cost-effective and can be powered by electricity from solar panels. The reaction also is easy to scale up. In addition, the team was able to demonstrate that the procedure can be performed in a continuous flow reactor.
- Electrochemical and Scalable Dehydrogenative C(sp3)–H Amination via Remote Hydrogen Atom Transfer in Batch and Continuous Flow,
Pavlo Nikolaienko, Marc Jentsch, Ajit P. Kale, Yunfei Cai, Magnus Rueping,
Chem. Eur. J. 2019, 25, 7177–7184.
https://doi.org/10.1002/chem.201806092
![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
