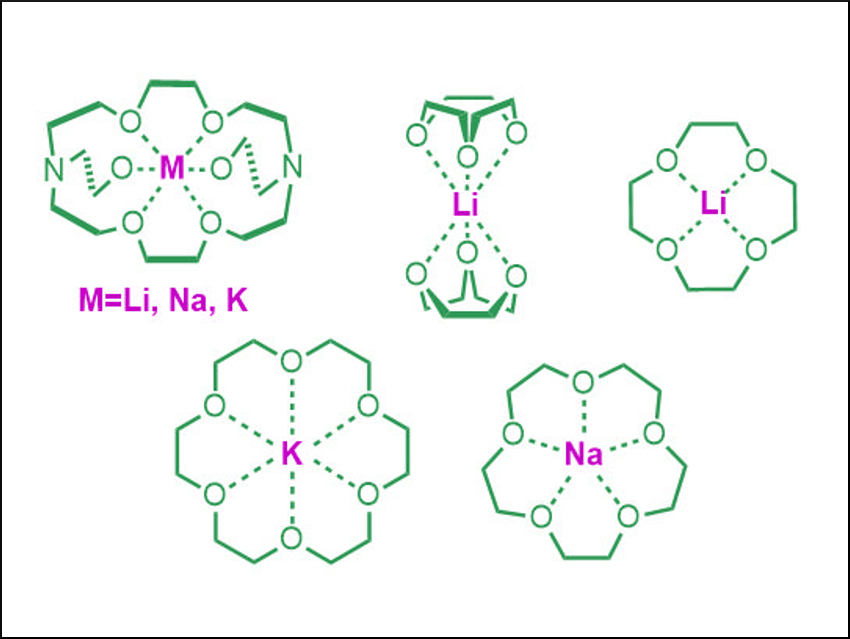
Alkali metals have the lowest ionization potentials among all the elements in the periodic table. However, due to collective effects, some molecules can show even lower ionization potentials. Compounds that beat the barrier of 3.9 eV are called superalkalis. Combining them with various anionic species opens the way to producing new materials with useful properties.
Alexander I. Boldyrev, Utah State University Logan, USA, and colleagues have performed density functional theory (DFT) calculations on alkali metals complexes with crown ethers and cryptands (pictured). The team found extremely low ionization potentials for [M([2.2.2]crypt)] (M=Li, Na, K). The ionization energies are significantly lower than those of alkali metal atoms. Thus, the investigated complexes can be defined as superalkalis. The [K([2.2.2]crypt)] complex (pictured top left) has a record low ionization potential of only 1.52 eV.
The ionization energy decreases based on the metal in the row Li‐Na‐K. Mono‐crown-ether complexes have higher ionization energies than more highly coordinated species. The results point to a new direction for the design of chemical species with very low ionization potentials.
- Record Low Ionization Potentials of Alkali Metal Complexes with Crown Ethers and Cryptands,
Nikolay V. Tkachenko, Zhong-Ming Sun, Alexander I. Boldyrev,
ChemPhysChem 2019.
https://doi.org/10.1002/cphc.201900422