Water-soluble nanocrystals, such as those made of sodium chloride (NaCl), are ideal templates for the fabrication of colloidal nanoparticles with hollow interiors. The templates can be easily removed by simply dispersing the core-shell samples in water. However, it has been challenging to produce nanocrystals of water-soluble compounds with uniform and controllable sizes.
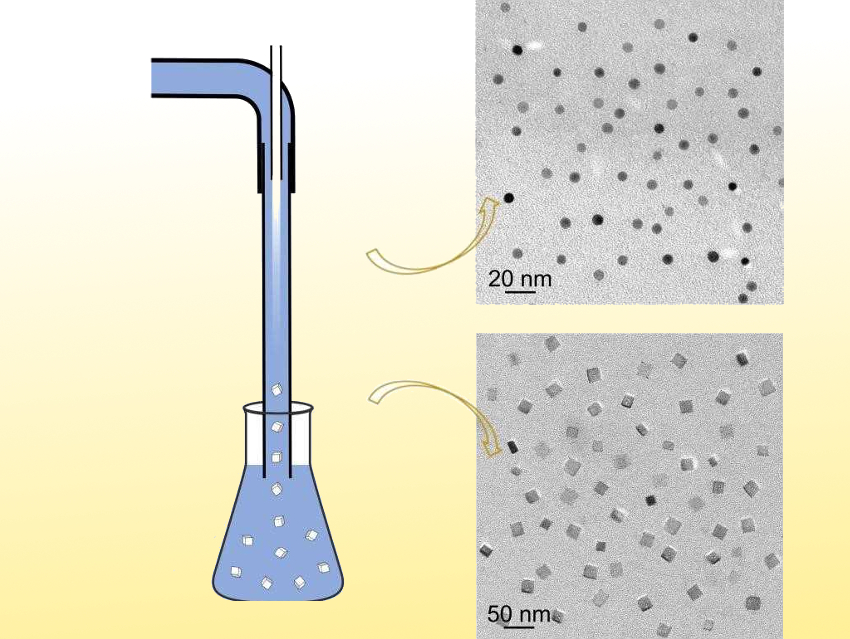
Younan Xia, Georgia Institute of Technology and Emory University, Atlanta, USA, and colleagues have developed a simple method for the continuous and scalable production of NaCl nanocrystals. The team used so-called anti-solvent precipitation in a fluidic device (pictured). They used an aqueous NaCl solution together with an ethanol phase as the anti-solvent. Then they combined the two phases using a coaxial flow system (i.e., nested tubes). Upon contact between the two phases, NaCl nanocrystals are precipitated out at the interface.
By regulating the hydrodynamic parameters during the mixing process, the researchers can obtain NaCl nanocubes and nanospheres as small as 20 nm and 6 nm, respectively (pictured). They also were able to generate KCl nanocrystals with uniform and controllable sizes using a similar approach. This demonstrates the generality of this method for the production of water-soluble nanocrystals.
- Continuous Production of Water-Soluble Nanocrystals through Anti-Solvent Precipitation in a Fluidic Device,
Qiaoshan Chen, Zachary D. Hood, Jichuan Qiu, Baohong Guan, Younan Xia,
ChemNanoMat 2019.
https://doi.org/10.1002/cnma.201900338