High-performance electrocatalysts are critical for many sustainable energy technologies, including water electrolysis, fuel cells, and metal-air batteries. The development of stable, economically viable catalysts is still a challenge, particularly if complex proton-coupled multielectron‐transfer reactions, such as water oxidation or oxygen reduction, need to be catalyzed.
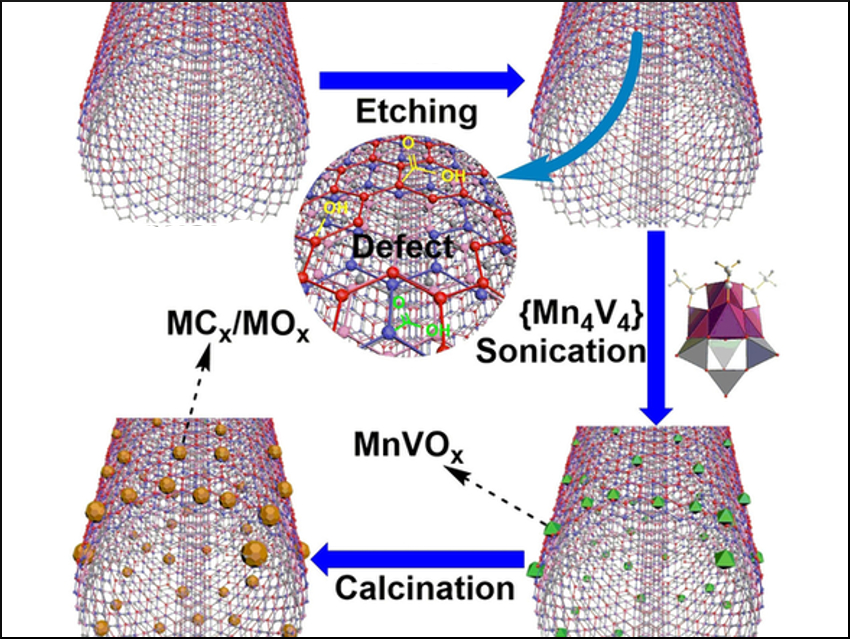
Carsten Streb, Rongji Liu, Ulm University and Helmholtz-Institute Ulm, Germany, and colleagues have reported a new class of composite electrocatalysts. The researchers used a sonication-driven synthesis to deposit the precursor [Mn4V4O17(OAc)3]3– onto etched multiwalled carbon nanotubes (MWCNTs). Then, a thermal treatment gave nanostructured manganese vanadium oxides/carbides, which are strongly linked to the MWCNTs (pictured bottom left).
The composite catalyst has an outstanding performance and long-term stability for the oxygen reduction reaction (ORR) and the oxygen evolution reaction (OER) in alkaline aqueous media. In addition, high activities for the oxygen evolution in acidic electrolytes and for the evolution of chlorine from aqueous hydrochloric acid were observed. According to the team, the same protocol could be used for the designing of new mixed‐metal carbides/oxides as (electro‐)catalysts for other challenging multielectron‐transfer reactions.
- Transition-Metal Oxides/Carbides@Carbon Nanotube Composites as Multifunctional Electrocatalysts for Challenging Oxidations and Reductions,
Xiaolin Xing, Rongji Liu, Kecheng Cao, Ute Kaiser, Carsten Streb,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201901400