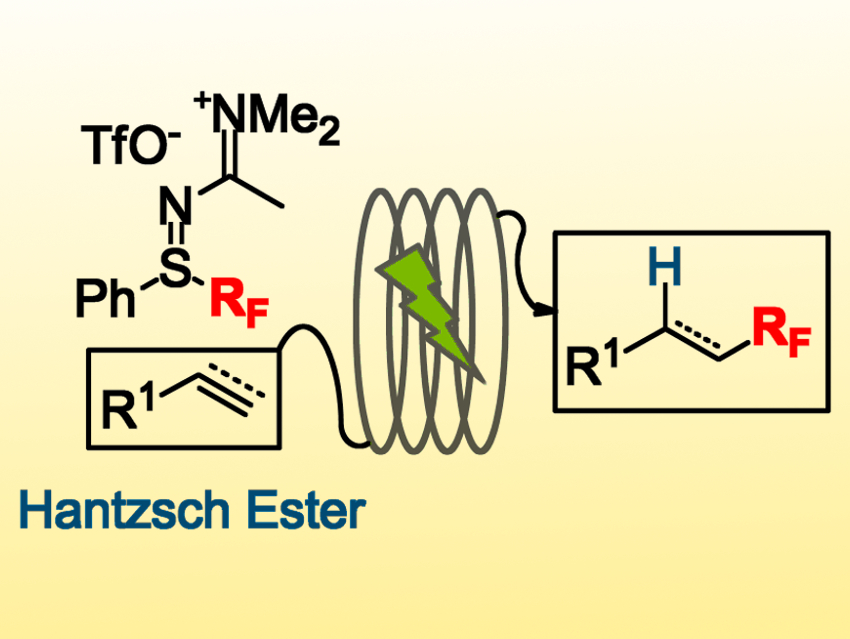
The introduction of perfluoroalkyl groups, such as trifluoromethyl, into a molecule changes its properties—often in useful ways. Organofluorine chemistry has applications in, e.g., materials science and pharmaceutical chemistry. The development of bench-stable, environmentally friendly, and selective perfluoroalkylating reagents is, thus, important.
Anne-Laure Barthelemy, Guillaume Dagousset, and Emmanuel Magnier, Université de Versailles‐Saint‐Quentin, France, have developed a protocol for the functionalization of various alkenes and alkynes with perfluoroalkyl groups (C4F9, CF3, CF2Br, CFCl2). This hydroperfluoroalkylation process uses sulfilimino iminium salts (pictured top left), which can be easily prepared on a large scale, as the source of fluorinated radicals and a Hantsch ester as the hydrogen-atom source. Rhodamine B is used as a metal-free photocatalyst, which is activated under green LED irradiation.
The reaction is performed in a continuous-flow process under mild conditions. The alkenes or alkynes are converted to desired perfluoroalkylated alkanes or alkenes in moderate to good yields. The process is efficient and the reaction has good functional group tolerance. According to the team, this is the first time alkynes have been used as substrates in a photoredox-catalyzed trifluoromethylation with a flow process.
- Metal-Free Visible-Light-Mediated Hydrotrifluoromethylation of Unactivated Alkenes and Alkynes in Continuous Flow,
Anne-Laure Barthelemy, Guillaume Dagousset, Emmanuel Magnier,
Eur. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201901252